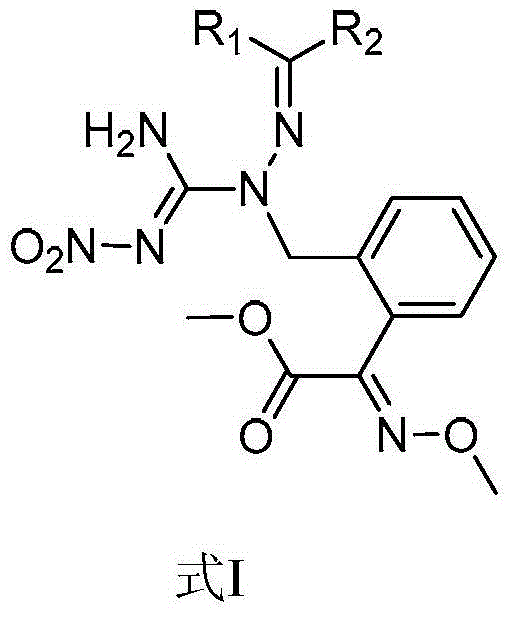
Methoxyimino phenylacetate compounds containing nitrohydrazinecarboximidamide structures as well as preparation method and application of methoxyimino phenylacetate compounds
A compound, phenethyl technology, applied in the field of methoxyiminophenylacetate compounds and their preparation, can solve the problems of increased drug residues, cumbersome spraying operations, and increased cost of spraying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
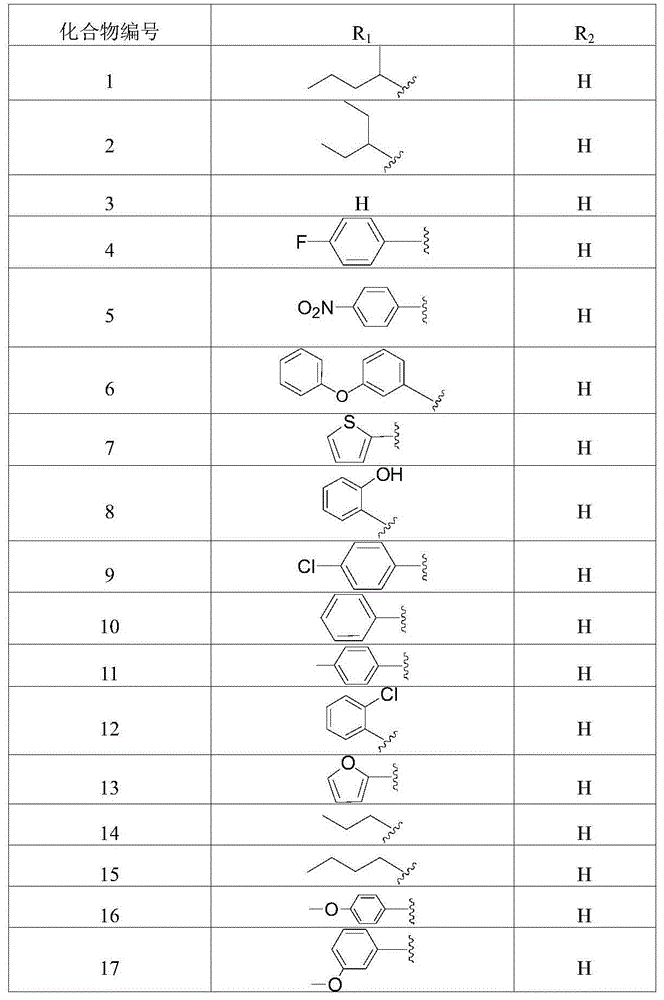
[0066] Example 1, 2-(2-((1-(N′-nitroamidino)-2-pentylidene)hydrazinomethyl)-phenyl)-(E)-2-methoxyimino Preparation of methyl acetate (compound 15)
[0067] (1) Preparation of 1-nitro-3-aminoguanidine
[0068] Put a 100mL three-neck bottle in an oil bath, and put in 2.0g (19.2mmol) of nitroguanidine and 25mL of water in sequence. While heating to 55° C. under magnetic stirring, 1.3 g (21.6 mmol) of 85% hydrazine hydrate was slowly added dropwise from the dropping funnel. Keep the material temperature at 55°C and continue the reaction for 15 minutes. When the nitroguanidine solid dissolves and turns into an orange-yellow clear liquid, quickly cool it in an ice-salt water bath, and slowly add concentrated hydrochloric acid dropwise to adjust the pH to 5-6, and continue to cool in an ice-salt water bath overnight. Filter under reduced pressure and dry naturally in a fume hood. Recrystallized with water to obtain 1.6 g of light yellow crystals, yield 70%, melting point 191-192°...
Embodiment 2
[0080] Example 2, 2-(2-((1-(N'-nitroamidino)-2-(4-nitrobenzylidene)hydrazinomethyl)-phenyl)-(E)-2 -Methoxyimine
[0081] Preparation of methyl glycolate (compound 5)
[0082] (1) Preparation of 1-nitro-4-(4-nitrobenzaldehyde) aminoguanidine
[0083] Into a 250mL three-neck flask, 11.9g (0.1mol) of 1-nitro-3-aminoguanidine, 100mL of anhydrous methanol and 0.6mL of glacial acetic acid were successively added. Heating to boiling under magnetic stirring, slowly added 9.5 g (0.11 mol) of p-nitrobenzaldehyde and 10 mL of anhydrous methanol solution dropwise from the dropping funnel. After the dropwise addition was completed, the mixture was heated to reflux and reacted at reflux for 3.5 hours. Cool down, pour into 150mL of ice water, solid precipitates out, let stand overnight. The obtained crude product was recrystallized from ethyl acetate to obtain a yellow solid with a yield of 95% and a melting point of 264-266°C.
[0084] The structural characterization data are as follow...
Embodiment 3
[0091]Example 3.2-(2-((1-(N′-nitroamidino)-2-(isopropylidene)hydrazinomethyl)-phenyl)-(E)-2-methoxyimino Acetic acid
[0092] Preparation of methyl ester (compound 28)
[0093] (1) Preparation of 1-nitro-4-acetone aminoguanidine
[0094] In a 250mL three-necked flask, 11.9g (0.1mol) of 1-nitro-3-aminoguanidine, 18.4g (0.4mol) of acetone, 100mL of anhydrous methanol and 2mL of concentrated hydrochloric acid were successively added. Heat to reflux for 5 hours with magnetic stirring. Stand to cool, concentrate the solution to saturation, a large number of colorless crystals precipitate out, stand overnight, filter with suction, rinse with water:alcohol=2:1 solution to obtain 11.5g of white solid, yield 80%, melting point 164-166°C .
[0095] The structural characterization data are as follows:
[0096] 1.92(s,3H,CH 3 ),1.99(s,3H,CH 3 ), 7.99 (brs, 1H, =NNH-), 8.64 (brs, 1H, -NH-NO 2 ), 10.76 (s, 1H, -C=NH).
[0097] (2) 2-(2-((1-(N′-nitroamidino)-2-(isopropylidene)hydraz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com