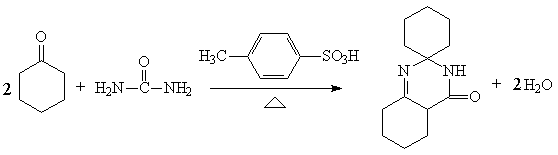
Preparation method of 6-cyclohexyl-3,4-cyclohexyl-5-nitrocaprolactam-4-ene
A nitrogen caprolactam and cyclohexyl technology is applied in the field of synthesis of pharmaceutical intermediates, which can solve the problems of flushing materials, easy occurrence of safety accidents and the like, and achieves the effects of high yield, low cost and moderate reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1. Under normal pressure, add 200g of cyclohexanone and 40ml of toluene into a 1000ml flask equipped with a water separator and a condenser, then start stirring, raise the temperature to 135-145°C and reflux for dehydration, and separate the water from the water separator , and then through a triangular funnel with a branch pipe inner diameter of 4mm, continuously add 30g of urea within 1 hour, after adding, maintain the reaction for 1.5 hours, remove 18ml of water, stop heating, add 0.1g of spirocycle crystals to induce crystallization, and then Slowly lower to 20°C, then filter with suction, wash the filter cake with 100g cyclohexanone, the wet weight of the filter cake is 77.5g, the dry weight is 63.1g, the purity is 97.5%, the calculated molar yield is 57.4%, the filter cake can be directly put into the water without drying The first step is the hydrolysis process, and the mother liquor and lotion are put into the next batch of synthesis reaction.
example 2
[0021] Example 2. Under normal pressure, add 260g of the previous batch of mother liquor and lotion into a 1000ml flask equipped with a water separator and a condenser, then start stirring, raise the temperature to 135-145°C for reflux dehydration, and separate out the water separator Water, then through a triangular funnel with a branch pipe inner diameter of 4mm, continuously add 30g of urea within 1 hour, after the addition, maintain the reaction for 1.5 hours, remove 18.5ml of water, stop heating, add 0.1g of spirocycle crystals to induce crystallization , then slowly lowered to room temperature, then suction filtered, washed the filter cake with 100g cyclohexanone, the wet weight of the filter cake was 111.8g, the dry weight was 95.5g, the purity was 97.3%, and the calculated molar yield was 86.8%. The filter cake can be directly put into In the next step of hydrolysis process, the mother liquor and lotion are put into the next batch of synthesis reaction.
example 3
[0022] Example 3. Under normal pressure, add 270g of the previous batch of mother liquor and lotion to a 1000ml flask equipped with a water separator and a condenser, then start stirring, raise the temperature to 135-145°C for reflux dehydration, and separate the water separator Water, then through a triangular funnel with a branch pipe inner diameter of 4mm, continuously add 30g of urea within 1 hour, after the addition, maintain the reaction for 1.5 hours, remove 17ml of water, stop heating, add 0.1g of spirocycle crystals to induce crystallization, Then slowly drop to room temperature, then suction filter, wash the filter cake with 100g cyclohexanone, the wet weight of the filter cake is 112.9g, the dry weight is 88.9g, the purity is 97.2%, and the calculated molar yield is 80.8%. The first step is the hydrolysis process, and the mother liquor and lotion are put into the next batch of synthesis reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com