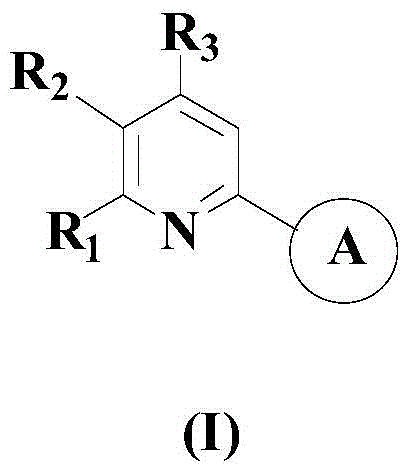
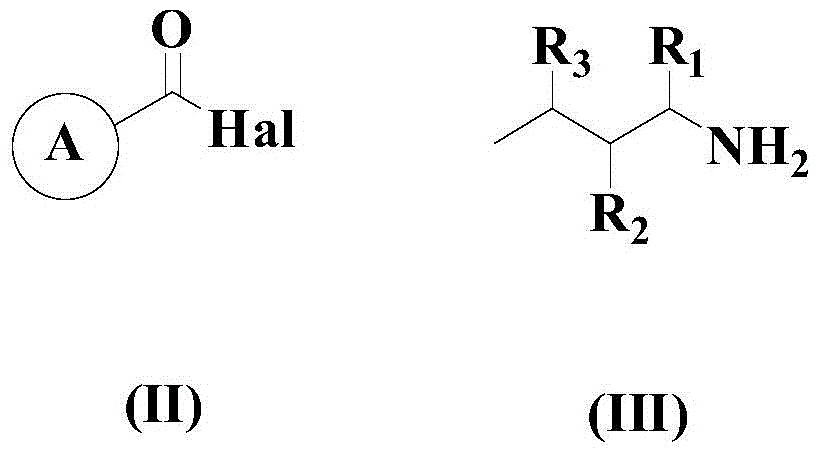
A kind of synthetic method of 2-substituted pyridine drug intermediate compound
A synthetic method and compound technology, which is applied in the synthesis of pharmaceutical intermediate compounds and the synthesis of 2-substituted pyridine drug intermediate compounds, which can solve the problem of unsatisfactory reaction yield and rare synthetic methods of 2-substituted pyridine compounds and other issues, to achieve the effect of good industrial application prospects and value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: 2-(3,4-difluorophenyl)pyridine
[0046]
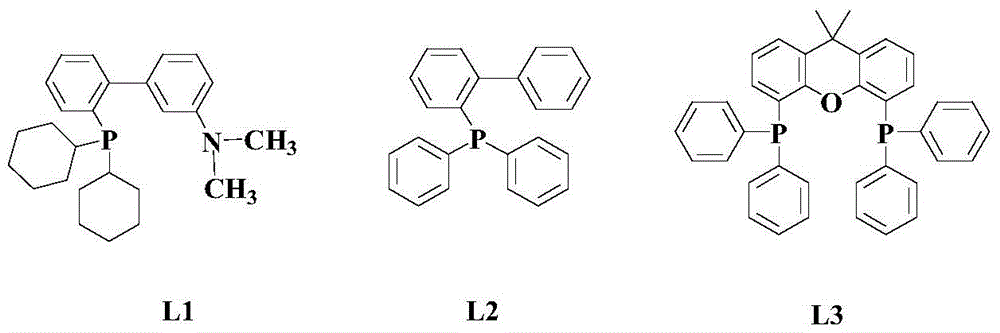
[0047] At room temperature, add 100mmol 3,4-difluorobenzoyl chloride, 100mmol n-butylamine, 5mmol copper(II) ethylacetoacetate, 5mmol ligand L1, 100mmol dimethylamino to an appropriate amount of n-propanol in the reactor Pyridine and 20 mmol of silver acetate were heated up to 60°C with stirring, and the reaction was continued with stirring at this temperature for 12 hours.
[0048] After the reaction, the reaction system was naturally cooled to room temperature, fully washed with deionized water, separated into layers, the upper organic phase was taken, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the resulting residue was separated by silica gel column chromatography. Deliquification is a mixture of n-butanol and ethyl acetate, the volume ratio of the two is 1:3,
[0049] The compound 2-(3,4-difluorophenyl)pyridine of formula (I) was obtained with a yield of 96.2%.
[0050] 1 H...
Embodiment 2
[0052] Example 2: 2-p-nitrophenylpyridine
[0053]
[0054] Add 100mmol p-nitrobenzoyl chloride, 150mmol n-butylamine, 10mmol copper(II) ethylacetoacetate, 8mmol ligand L1, 150mmol dimethylaminopyridine and 40mmol silver acetate to an appropriate amount of toluene in the reactor at room temperature , the temperature was raised to 80°C with stirring, and the stirring reaction was continued at this temperature for 10 hours.
[0055] After the reaction, the reaction system was naturally cooled to room temperature, fully washed with deionized water, separated into layers, the upper organic phase was taken, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the resulting residue was separated by silica gel column chromatography. Deliquoring is a mixture of n-butanol and ethyl acetate, the volume ratio of the two is 1:4, and the compound 2-p-nitrophenylpyridine of formula (I) is obtained with a yield of 95.8%.
[0056] 1 H-NMR (400MHz, CDCl 3 )δ:8.76...
Embodiment 3
[0058] Example 3: 2,2'-bipyridine
[0059]
[0060] At room temperature, add 100 mmol of pyridin-2-ylformyl chloride, 200 mmol of n-butylamine, 15 mmol of copper(II) ethylacetoacetate, 10 mmol of ligand L1, 200 mmol of dichloroethane to an appropriate amount of 1,2-dichloroethane in the reactor. Add aminopyridine and 60 mmol silver acetate, stir and heat up to 90° C., and keep stirring at this temperature for 8 hours.
[0061] After the reaction, the reaction system was naturally cooled to room temperature, fully washed with deionized water, separated into layers, the upper organic phase was taken, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the resulting residue was separated by silica gel column chromatography. The deliquored mixture is a mixture of n-butanol and ethyl acetate, the volume ratio of which is 1:5, and the compound 2,2'-bipyridine of formula (I) is obtained with a yield of 96.7%.
[0062] 1 H-NMR (400MHz, CDCl 3 )δ: 8.69 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com