A kind of synthetic method of water-soluble porphyrin and its photocatalytic application
A synthesis method and water-soluble technology, which is applied in the synthesis method of water-soluble porphyrin and its photocatalytic application field, can solve the problems of healthy cell toxicity and side effects, long residence time, poor solubility, etc. The effect of high purity and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
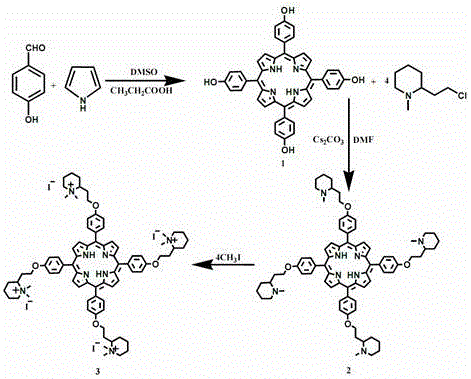
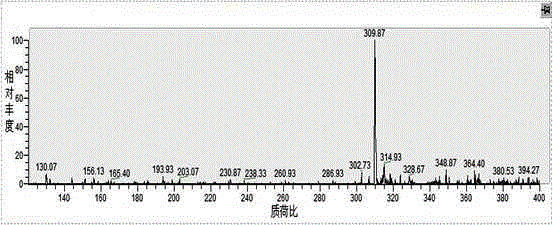
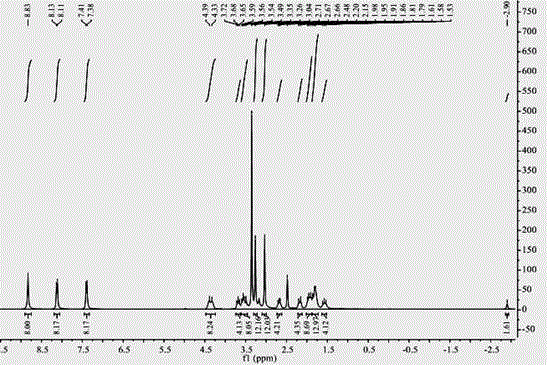
[0026] A kind of synthetic method of water-soluble porphyrin, the compound name of described water-soluble porphyrin is tetraiodide 5,10,15,20-tetra{4-[2-(N,N-dimethyl-2-piper Pyridine) ethoxy] phenyl} porphyrin, the steps are as follows:
[0027] 1) Synthesis of 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin
[0028] Dissolve 6.1 g of benzaldehyde in 110 mL of propionic acid a, add 6 mL of dimethyl sulfoxide (DMSO) catalyst, stir mechanically, and then add 3.5 mL of freshly distilled pyrrole and 10 mL of propionic acid dropwise when heated to 128°C under reflux The mixed solution of b, 1 drop per second, heated up to 141°C, refluxed for 2 hours, suction filtered while it was hot, washed the filter cake twice with propionic acid, and obtained blue solid 5,10,15,20-tetrakis(4-hydroxybenzene Base) porphyrin crude product, drying stand-by;
[0029] Put 10 g of the dried crude product above into a 100 mL round-bottomed flask, add 20 mL of absolute ethanol and heat to reflux, stop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com