A kind of method of synthesizing blastamide
A technology of blastamide and toluene, applied in the field of synthesizing blastamide, can solve problems such as unfavorable popularization and application, long reaction time, complex process, etc., and achieve the effects of reducing raw material types, shortening reaction cycle, and simplifying the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
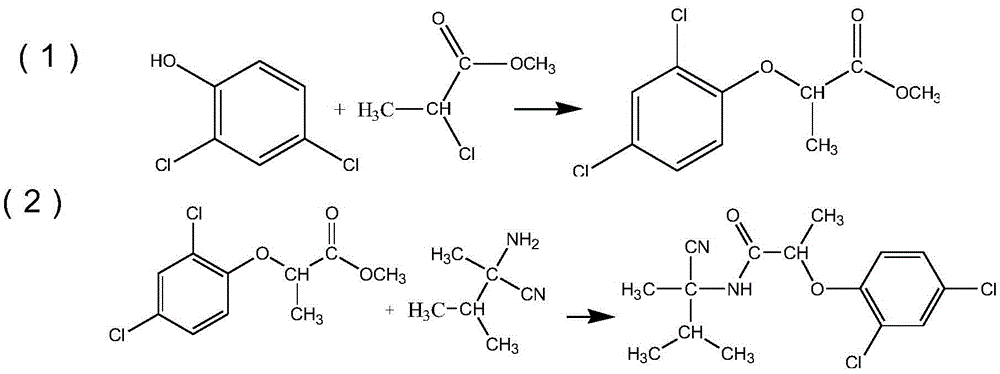
[0018] 163g (1mol) of 2,4-dichlorophenol, 1L of toluene, 40g (1mol) of sodium hydroxide and 122.5g (1mol) of methyl 2-chloropropionate were added to the 2L reaction flask, and the temperature was raised to 55°C for reaction 8 Hour, the toluene phase is washed with water after the reaction is finished, and the toluene phase after the washing is carried out to distill the solvent under reduced pressure to obtain methyl propionate;
[0019] 112g (1mol) of 2-amino-2,3-dimethylbutyronitrile, 550ml of toluene and 84g (1mol) of sodium bicarbonate and the methyl propionate obtained by the reaction were added to the 1L reaction flask, and the temperature was controlled at 15°C to carry out The reaction was carried out for 6 hours, the toluene phase was washed with water after the reaction, and the toluene phase after the washing was carried out to distill the solvent under reduced pressure, and then recrystallized with a mixed solvent of 1.1 L of water and ethanol, and the volume ratio ...
Embodiment 2
[0021] 163g (1mol) of 2,4-dichlorophenol, 1.2L of toluene, 40g (1mol) of sodium hydroxide and 134.8g (1.1mol) of methyl 2-chloropropionate were added to a 2L reaction flask, and the temperature was raised to 60°C The reaction was carried out for 9 hours, the toluene phase was washed with water after the reaction, and the toluene phase after the washing was carried out under reduced pressure distillation solvent to obtain methyl propionate;
[0022] 112g (1mol) of 2-amino-2,3-dimethylbutyronitrile, 500ml of toluene and 84g (1mol) of sodium bicarbonate and methyl propionate obtained by the reaction were added to the 1L reaction flask, and the temperature was controlled at 10°C to carry out The reaction was carried out for 5 hours, and the toluene phase was washed with water after the reaction, and the toluene phase after the washing was carried out to distill the solvent under reduced pressure, and then recrystallized with a mixed solvent of 1 L of water and ethanol, and the volu...
Embodiment 3
[0024] 163g (1mol) of 2,4-dichlorophenol, 1.3L of toluene, 40g (1mol) of sodium hydroxide and 147g (1.2mol) of methyl 2-chloropropionate were added to the 2L reaction flask, and the temperature was raised to 45°C for reaction For 10 hours, the toluene phase was washed with water after the reaction, and the toluene phase after the water washing was subjected to reduced pressure distillation of the solvent to obtain methyl propionate;
[0025] Add 112g (1mol) of 2-amino-2,3-dimethylbutyronitrile, 550ml of toluene and 84g (1mol) of sodium bicarbonate and the methyl propionate obtained by the reaction to the 1L reaction flask, and the temperature is controlled at 8°C to carry out The reaction was carried out for 7 hours, and the toluene phase was washed with water after the reaction, and the toluene phase after the washing was carried out to distill the solvent under reduced pressure, and then recrystallized with a mixed solvent of 1.2 L of water and ethanol. The volume ratio of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com