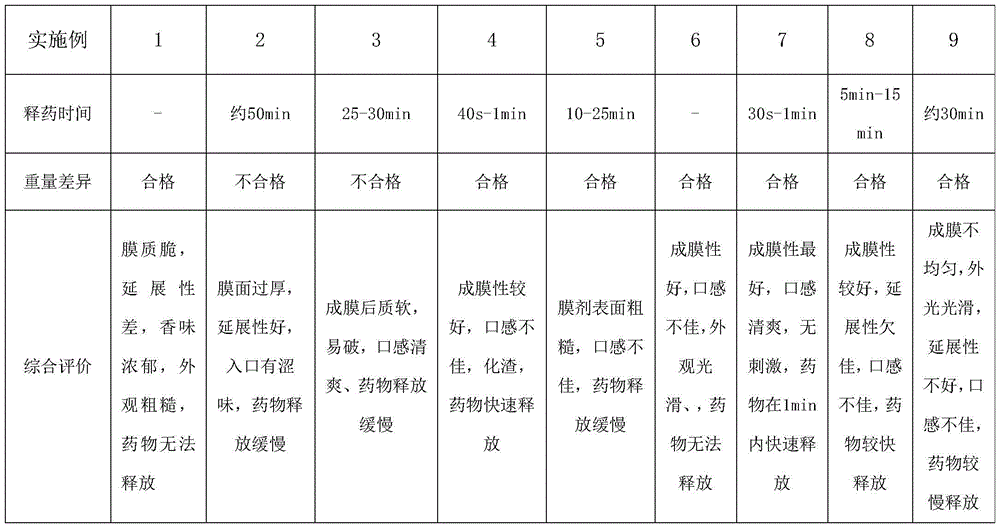
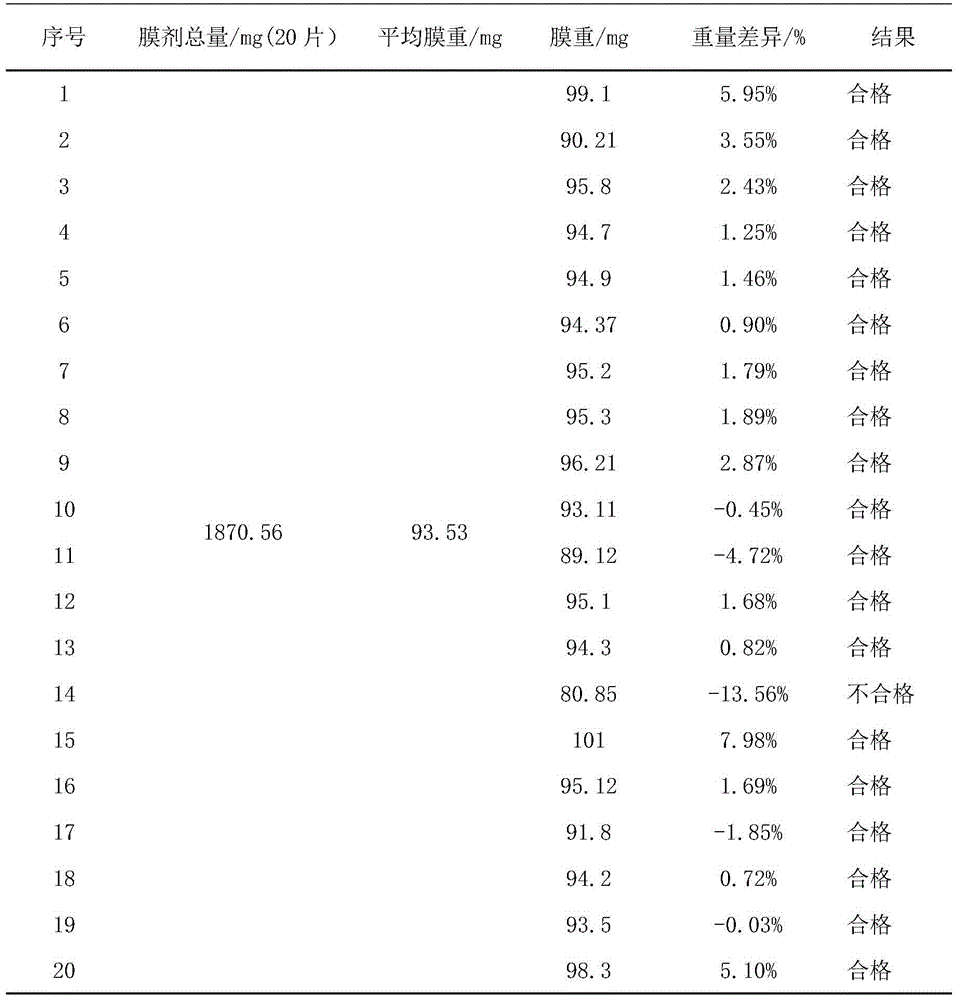
Immediate-release film of Estherglam
A technology of eseglan and film formulations, applied in the field of immediate release film formulations, to achieve the effects of improving compliance, convenient use and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
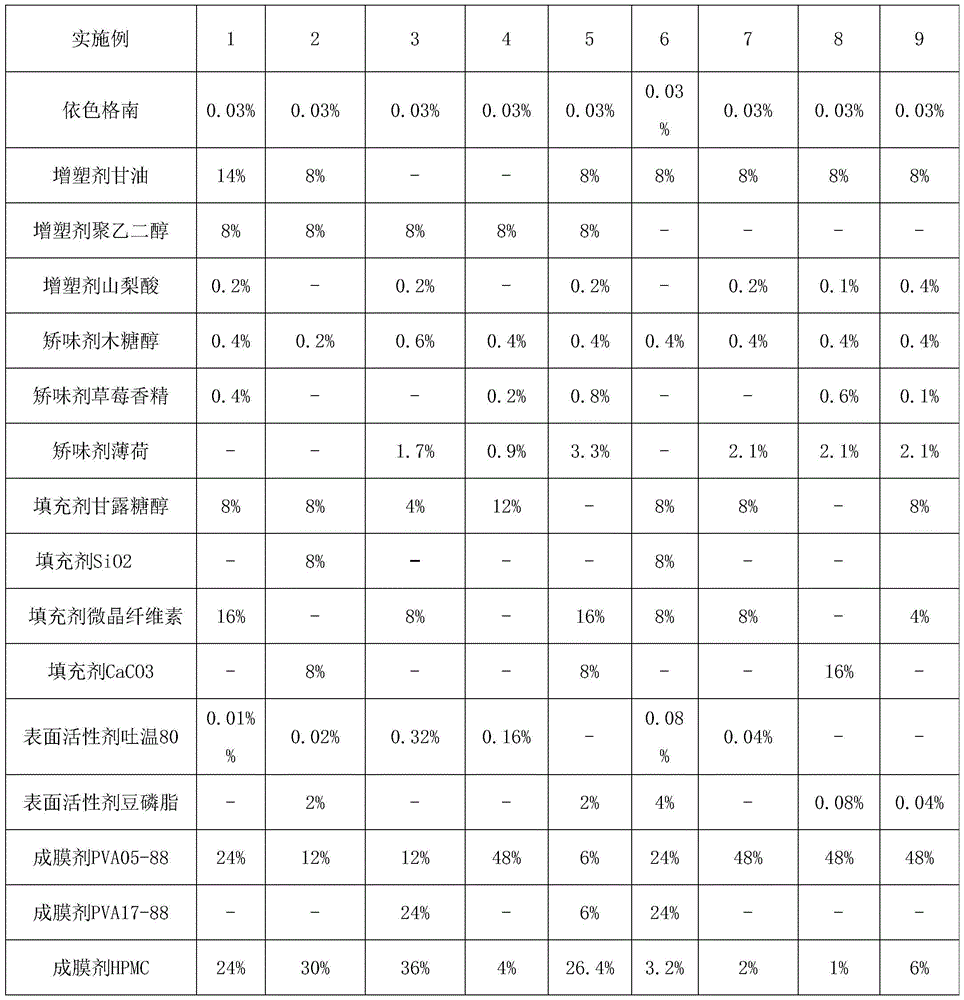
[0027] Embodiment 1 The preparation of quick-release film of the present invention (each embodiment prescription is as the criterion with Table 1)
[0028] Immediate Release Film Prescription
[0029] Estherglam 0.03% Glycerin 14% Polyethylene Glycol 8% Sorbic Acid 0.2%
[0030] Xylitol 0.4%, Strawberry Flavor 0.4%, Mannitol 8%, Microcrystalline Cellulose 16%
[0031] Tween 80 0.01% PVA05-88 24% HPMC 24%
[0032] The balance is phosphate buffer (PH6.8)
[0033] Preparation method:
[0034] 1. Soak PVA05-88 in enough buffer solution to make it fully swell, heat the fully swollen PVA05-88 at 65°C-80°C for 2 hours to dissolve, and filter;
[0035] 2. Mixing: Slowly add other excipients except the flavoring agent into the dissolved PVA05-88, stir while adding, then add the flavoring agent and stir slowly until the solution cools to 40-44°C, then gradually add the Segnam, stirring while adding;
[0036] 3. Add buffer solution to make a sufficient amount, and stir well to make...
Embodiment 2
[0037] Embodiment 2 The preparation of quick-release film agent of the present invention
[0038] Immediate Release Film Prescription
[0039] Estherglam 0.03% Glycerin 8% Macrogol 8% Xylitol 0.2%
[0040] Mannitol 8% Silicon Dioxide 8% Calcium Carbonate 8% Tween 80 0.02%
[0041] Soy Lecithin 2% PVA05-88 12% HPMC 30%
[0042] The balance is phosphate buffer (PH6.8)
[0043] The preparation method is the same as in Example 1.
Embodiment 3
[0044] Embodiment 3 Preparation of immediate-release film of the present invention
[0045] Immediate Release Film Prescription
[0046] Estherglam 0.03% Macrogol 8% Sorbic Acid 0.2%
[0047] Xylitol 0.6% Peppermint 1.7% Mannitol 4% Microcrystalline Cellulose 8%
[0048] Tween 80 0.32% PVA05-88 12% PVA17-88 24%
[0049]HPMC 36% balance is phosphate buffer (PH6.8)
[0050] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com