Method for preparing acetyenic ketone by coupling carboxylate triazinyl ester with terminal alkyne
A technology of carboxylate triazine ester and terminal alkyne, which is applied in the field of synthesis of acetylenic compounds, can solve the problems of difficult substrates, etc., and achieve the effects of improving electrophilicity, using less catalyst, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
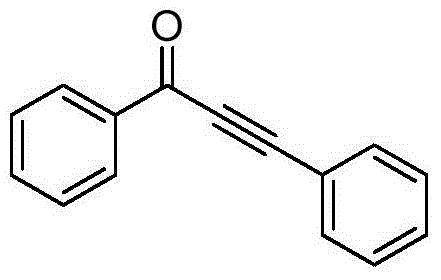
[0015] Taking the preparation of 1,3-diphenyl-2-ethynyl-1-one with the following structural formula as an example, the raw materials used and the preparation method are:
[0016]
[0017] Under the protection of nitrogen, add 0.0011g (0.005mmol) of palladium acetate and 0.1305g (0.5mmol) of triazine benzoate into the Shrek tube, vacuum three times with nitrogen, then add 55μL (0.6mmol) of phenylacetylene, 3mL of acetonitrile , stirred at 50°C for 10 hours, stopped the reaction, cooled down to room temperature naturally, filtered and separated by column chromatography to obtain white solid 1,3-diphenyl-2-ethynyl-1-one with a yield of 90%. The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (400MHz, CDCl 3 )δ: 8.15(d, J=7.5Hz, 2H), 7.65-7.51(m, 3H), 7.42(dd, J=17.3, 10.5Hz, 3H), 7.34(t, J=7.0Hz, 2H); 13 C NMR (101MHz, CDCl 3 )δ: 178.11, 137.04, 134.23, 133.18, 130.91, 129.6...
Embodiment 2
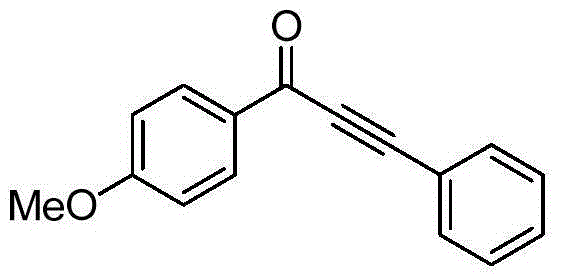
[0019] Taking the preparation of 1-(4-methoxyphenyl)-3-phenyl-2-ethynyl-1-one with the following structural formula as an example, the raw materials used and the preparation method are:
[0020]
[0021] In Example 1, the triazine benzoate used was replaced with equimolar triazine p-methoxybenzoate, and the other steps were the same as in Example 1 to prepare light grayish yellow solid 1-(4-methoxy Phenyl)-3-phenyl-2-ethynyl-1-one, its productive rate is 98%, and characteristic data is: 1 H NMR (400MHz, CDCl 3 )δ: 8.19(d, J=8.7Hz, 2H), 7.67(d, J=7.1Hz, 2H), 7.49-7.38(m, 3H), 6.98(d, J=8.8Hz, 2H), 3.89( s, 3H); 13 C NMR (101MHz, CDCl 3 )δ: 176.75, 164.61, 133.06, 132.08, 130.69, 130.45, 128.76, 120.49, 114.01, 92.39, 87.06, 55.70.
Embodiment 3
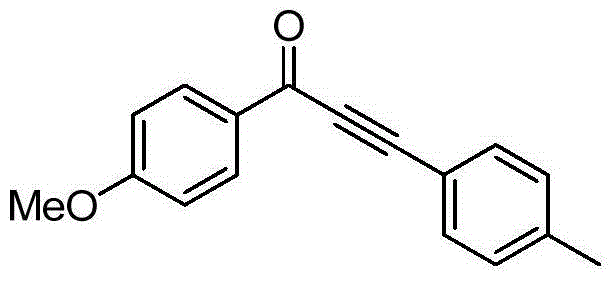
[0023] Taking the preparation of 1-(4-methoxyphenyl)-3-(4-methylphenyl)-2-ethynyl-1-one as an example with the following structural formula, the raw materials used and the preparation method are:
[0024]
[0025] In Example 2, the phenylacetylene used was replaced with equimolar p-methylphenylacetylene, and the other steps were the same as in Example 2 to prepare a yellow solid 1-(4-methoxyphenyl)-3-(4- Methylphenyl)-2-ethynyl-1-one, its productive rate is 98%, characterization data is: 1 H NMR (400MHz, CDCl 3 )δ: 8.19(d, J=8.9Hz, 2H), 7.57(d, J=8.1Hz, 2H), 7.22(d, J=7.9Hz, 2H), 6.98(d, J=8.9Hz, 2H) , 3.90(s, 3H), 2.40(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ: 176.87, 164.55, 141.41, 133.13, 132.07, 130.60, 129.58, 117.43, 114.00, 93.09, 86.93, 55.72, 21.87.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com