Copper sulfide flotation collector as well as preparation method and application thereof
A collector and copper sulfide technology, applied in flotation, solid separation, etc., can solve the problems of poor universality and weak collection ability of compound reagents, and achieve improved solid affinity and beneficial metal recovery rate High and selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of the copper sulfide flotation collector of the present invention is to generate a nucleophilic substitution reaction with an alkylamine, sodium hydroxide and carbon disulfide, and react for 1 to 2 hours at a temperature of 5 to 10°C to obtain sodium dithiocarbamate ; Sodium dithiocarbamate and alkyl chloroformate were reacted at 0~5°C for 2~4h to obtain the target collector alkoxycarbonyl alkyl dithiocarbamate.
[0021] The molar ratio of the alkylamine, sodium hydroxide and carbon disulfide is 1-1.05:1.05:4-6.
[0022] The molar ratio of the sodium dithiocarbamate to the alkyl chloroformate is 1:0.85-0.91.
[0023] Described alkylamine is dimethylamine, diethylamine or di-n-butylamine.
[0024] The sodium dithiocarbamate is sodium N,N-dimethyldithiocarbamate, sodium N,N-diethyldithiocarbamate or sodium N,N-dibutyldithiocarbamate.
[0025] The alkyl chloroformate is ethyl chloroformate, propyl chloroformate or butyl chloroformate.
[0026] Th...
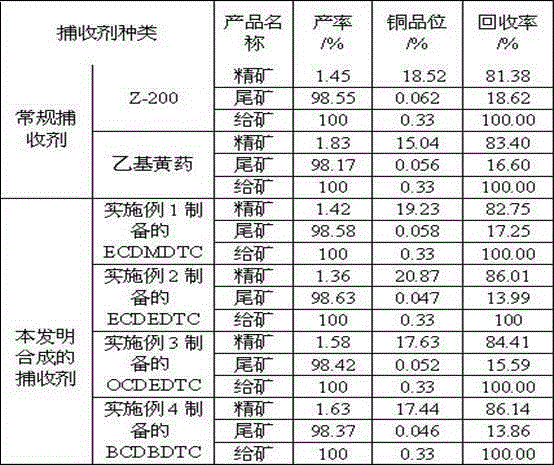
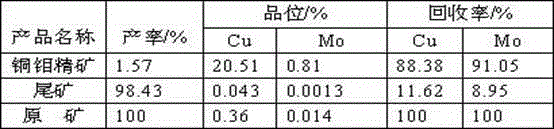
Embodiment 1
[0032] Example 1 Preparation of Ethoxycarbonyl Dimethyl Dithiocarbamate (ECDMDTC)
[0033] Add sodium hydroxide and carbon disulfide into the reaction flask at a molar ratio of 1.05:4~6, mechanically stir at 5~10°C and slowly add 1~1.05 parts of dimethylamine dropwise, and keep the temperature at 5~10°C after the dropwise addition React for 1~2 hours to obtain N,N-dimethyldithiocarbamate sodium with a yield of 88~92% based on dimethylamine, then slowly add 0.85~0.91 parts of ethyl chloroformate dropwise at 0~5°C After the dropwise addition, react at 0~5°C for 2~4 hours, filter to remove white solid sodium chloride, and distill the filtrate under reduced pressure at 50~65°C for 2~3 hours to remove impurities, and obtain yellow oily liquid ECDMDTC. The rate is 73~82%.
Embodiment 2
[0034] Example 2 Preparation of Ethoxycarbonyl Diethyl Dithiocarbamate (ECDEDTC)
[0035] Add sodium hydroxide and carbon disulfide to the reaction flask at a molar ratio of 1.05:6~9, mechanically stir at 5~10°C and slowly add 1~1.05 parts of diethylamine dropwise, and keep the temperature at 5~10°C after the dropwise addition React for 1~2 hours to obtain N,N-diethyldithiocarbamate sodium with a yield of 86~91% based on diethylamine, then slowly add 0.84~0.90 parts of ethyl chloroformate dropwise at 0~5°C , after the dropwise addition, react at 0~5°C for 2~4 hours, filter to remove white solid sodium chloride, and distill the filtrate under reduced pressure at 50~65°C for 2~3 hours to remove impurities, and obtain yellow oily liquid ECDEDTC. The rate is 72~81%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com