Separation and measurement method of besifloxacin hydrochloride and isomer of besifloxacin hydrochloride
A technology for besifloxacin hydrochloride and enantiomers, applied in the field of analytical chemistry, can solve the problems of complex pretreatment process, failure to find, poor operability, etc., and achieve simple sample pretreatment process, accurate separation and detection , the effect of strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Instruments and Conditions
[0029] American Agilent 1260 high performance liquid chromatography system and workstation; automatic sampling;
[0030]
[0031]
Embodiment 2
[0033] Experimental procedure
[0034] Diluent or blank solution is mobile phase A phase / B phase=55 / 45(V:V);
[0035] Take about 20 mg of besifloxacin hydrochloride bulk drug (prepared according to the method disclosed in Chinese patent CN 200880003118), accurately weigh it into a 10 mL brown measuring bottle, dissolve it with a diluent and dilute to the mark, shake well, and use it as the test solution.
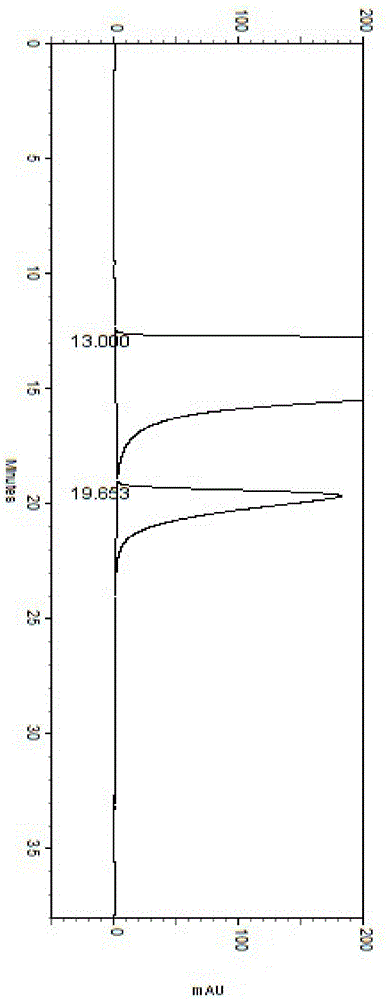
[0036] Get blank solution and need testing solution respectively, carry out high performance liquid chromatography analysis by the condition of embodiment 1, record chromatogram, the results are shown in figure 1 , figure 2 . figure 2 It shows that the separation and detection method of the present invention can separate besifloxacin hydrochloride and its enantiomers, and the high performance liquid chromatography shows that the separation degree is 3.56.
Embodiment 3
[0038] Take besifloxacin hydrochloride crude drug (prepared according to the method of CN 201310479217.0) about 20 mg of the test sample, accurately weigh it into a 10 mL brown volumetric flask, dissolve it ultrasonically with a diluent and dilute to the mark, shake well, and use it as the test sample solution.
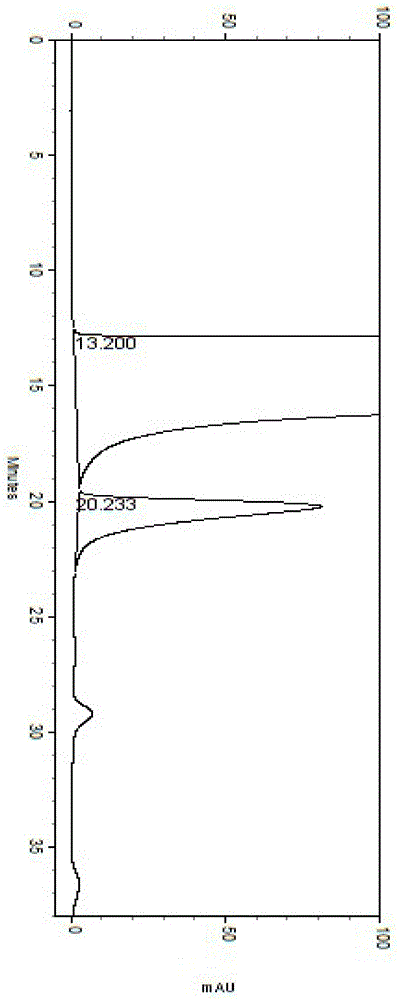
[0039] Get need testing solution, carry out high performance liquid chromatography analysis according to the condition of example 1, record chromatogram, the results are shown in image 3 .
[0040] image 3 It is proved that this method can be used for the quality monitoring of besifloxacin hydrochloride, and the high performance liquid chromatography shows that the resolution is 3.74.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com