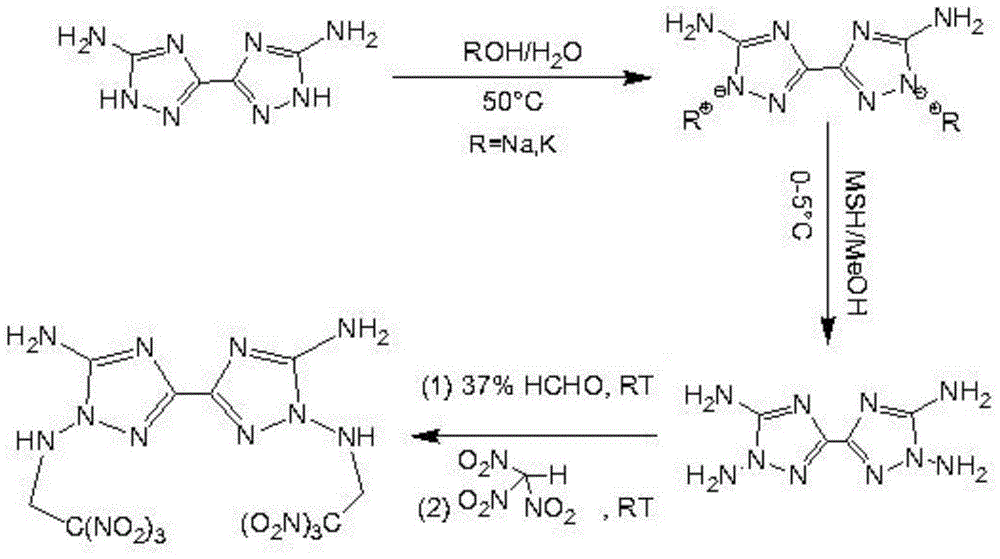
Energetic compound 3,3'-diamido-2,2'-2(2,2,2-trinitroethyl)-5,5'-linked triazole-diamine and preparation method and intermediate thereof
A technology of trinitroethyl and diamino is applied in the directions of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry, etc., and achieves the effects of good application prospects, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 30mL water to a 100mL three-necked flask, add 1g 6mmol of 3,3'-diamino-5,5'-bis(1H-1,2,4-triazole), add 0.67g 12mmol potassium hydroxide under stirring, Heat to 50°C to react for 0.5h, filter off the residue, spin dry the filtrate at a vacuum of 55mbar and a temperature of 60°C to obtain a light yellow solid, and obtain 3,3'-diamino-5,5'-bitriazole after vacuum drying -4,4'-dipotassium 1.33g, yield 91.7%, melting point 381.4°C.
[0035] In an ice-salt bath, add 1 g of 3,3'-diamino-5,5'-bitriazole-4,4'-dipotassium to 2,4,6-trimethylbenzenesulfonyl with a mass concentration of 82.4% Hydroxylamine in methanol solution, stirred for 6 hours, returned to room temperature, filtered under reduced pressure, washed with water, and dried in vacuum at 50°C to obtain the intermediate 3,3',4,4'-tetraamino-5,5'- Bitriazole 0.64g, yield 81%. Water recrystallization is dark yellow crystal, melting point 78.1 ℃.
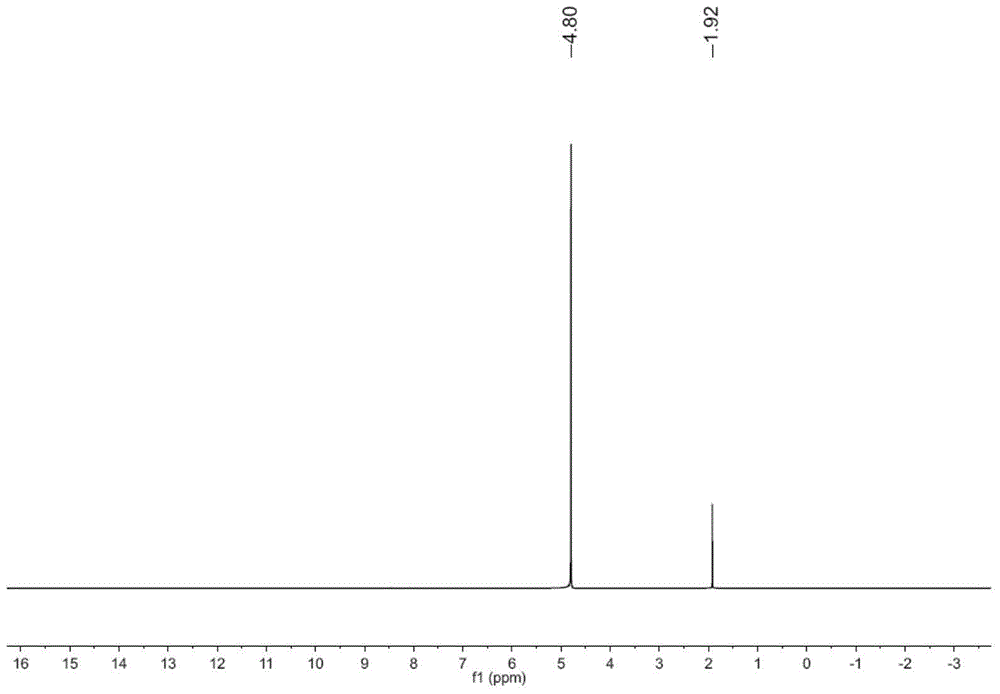
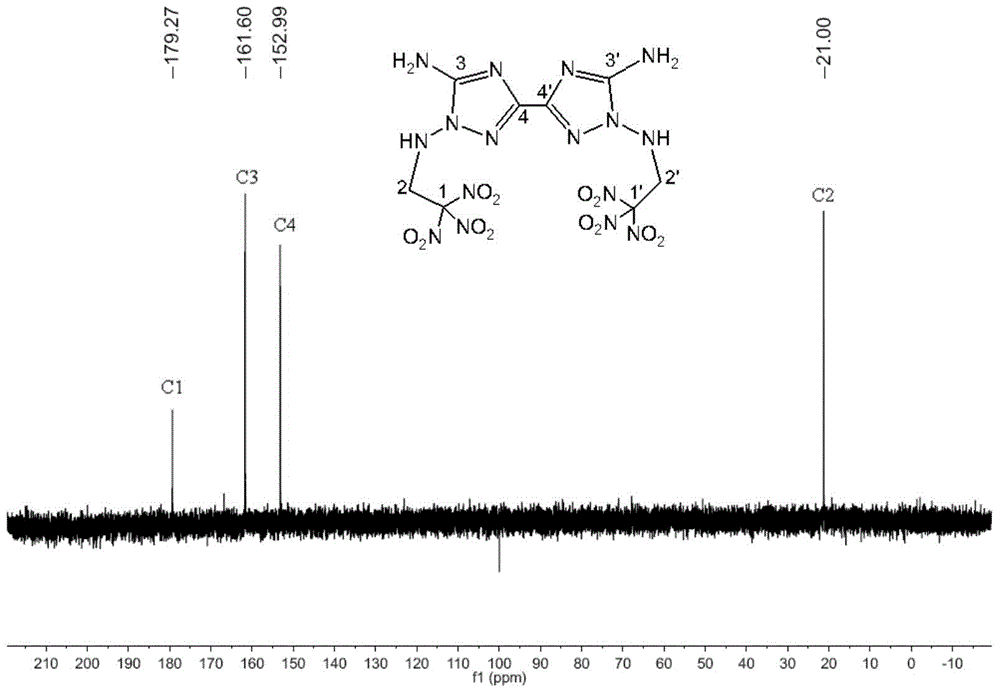
[0036] 3,3’,4,4’-tetraamino-5,5’-bitriazole 1 H NMR spectrum such ...
Embodiment 2
[0042] Add 20mL water into a 100mL three-necked flask, add 1g 6mmol of 3,3'-diamino-5,5'-bis(1H-1,2,4-triazole), add 0.67g 12mmol potassium hydroxide under stirring, Heat to 50°C for 1 hour, filter off the residue, spin dry the filtrate at a vacuum of 60mbar and a temperature of 45°C to obtain a white solid, and obtain 3,3'-diamino-5,5'-bitriazole-4 after vacuum drying ,4'-dipotassium 1.58g, yield 94.3%, melting point 357.0°C.
[0043] At 5°C, add 1 g of 3,3'-diamino-5,5'-bitriazole-4,4'-dipotassium to 90% mass concentration of 2,4,6-trimethylbenzenesulfonyl Hydroxylamine in methanol solution, stirred for 5 hours, returned to room temperature, filtered under reduced pressure, washed with water, and dried in vacuo at 45°C to obtain the intermediate 3,3',4,4'-tetraamino-5,5'- Bitriazole 0.69g, yield 86%. Water recrystallization is dark yellow crystal, melting point 78.0 ℃.
[0044] At room temperature, add 1 g of 3,3',4,4'-tetraamino-5,5'-bitriazole into a 38% mass concentrat...
Embodiment 3
[0046] Add 20mL water into a 100mL three-necked flask, add 1g 6mmol of 3,3'-diamino-5,5'-bis(1H-1,2,4-triazole), add 0.67g 12mmol potassium hydroxide under stirring, Heat to 50°C for 1 hour, filter off the residue, spin dry the filtrate at a vacuum of 60mbar and a temperature of 45°C to obtain a white solid, and obtain 3,3'-diamino-5,5'-bitriazole-4 after vacuum drying , 4'-dipotassium, the yield is 94.3%, and the melting point is 357.0°C.
[0047] At a temperature of 5°C, add 1 g of 3,3'-diamino-5,5'-bitriazole-4,4'-dipotassium to 2,4,6-trimethylbenzenesulfonyl with a mass concentration of 80% Hydroxylamine in methanol solution, stirred for 8 hours, returned to room temperature, filtered under reduced pressure, washed with water, and dried under vacuum at 45°C to obtain the intermediate 3,3',4,4'-tetraamino-5,5'- Bitriazole 0.67g, yield 85%. Water recrystallization is dark yellow crystal, melting point 78.1 ℃.
[0048] At room temperature, add 1 g of 3,3',4,4'-tetraamino-5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com