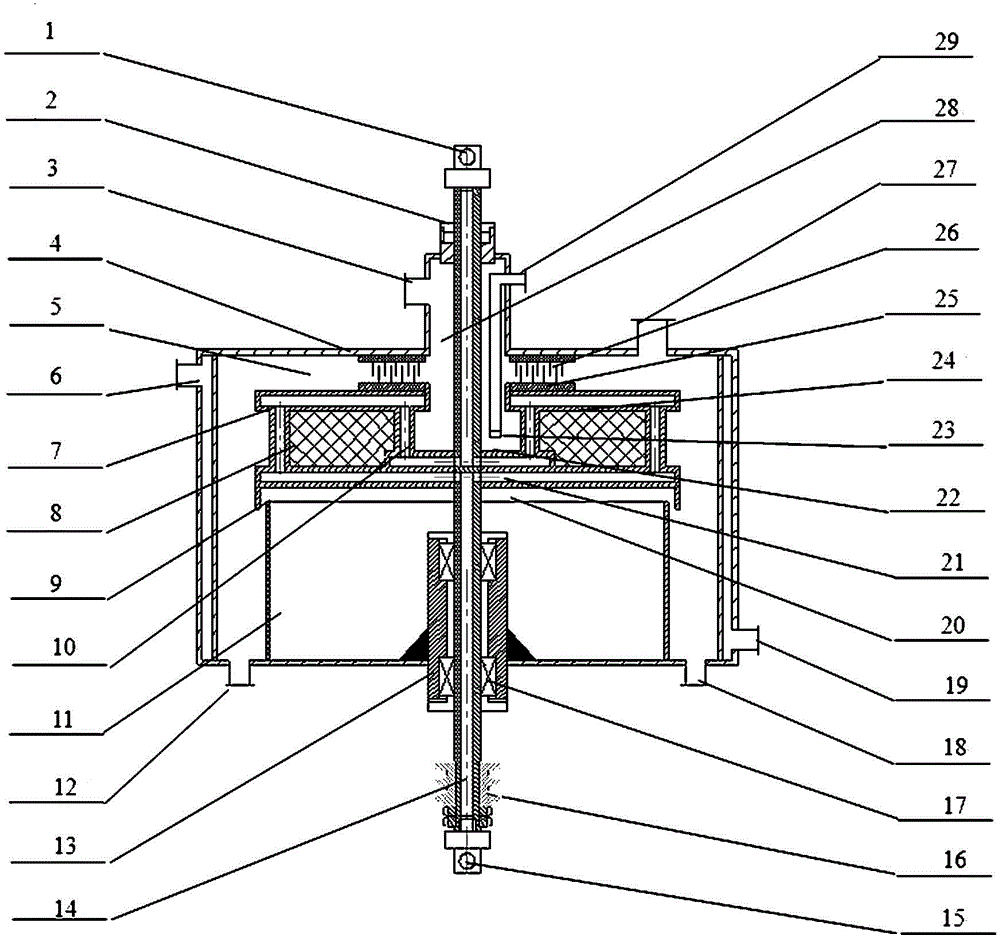
Sulfonation equipment and method for preparing p-toluenesulfonic acid by using gaseous sulfur trioxide sulfonated toluene
A technology of p-toluenesulfonic acid and sulfur trioxide, which is applied to the preparation of sulfonic acid, chemical instruments and methods, chemical/physical/physicochemical processes, etc. It can solve problems affecting product quality, no heat transfer structure, and small cooling area , to achieve the effects of stable product quality, improved mass transfer efficiency, and small equipment volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the sulfonation of toluene
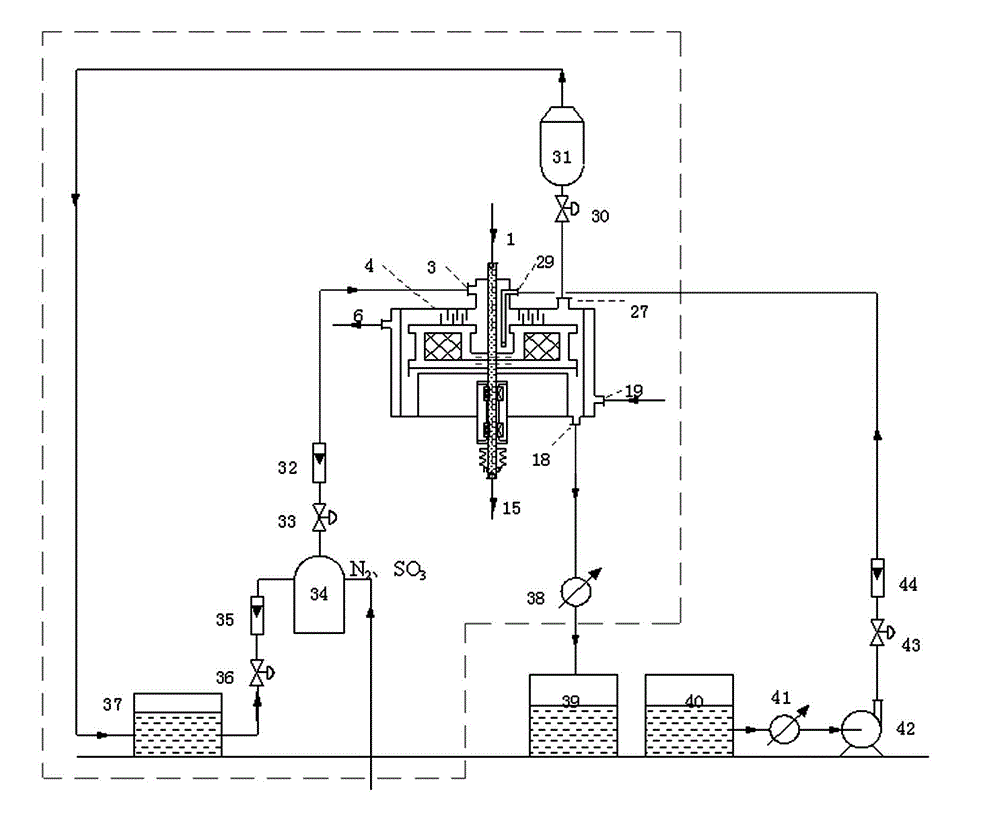
[0038] use figure 2 The plant scheme shown performs toluene sulfonation. Adjust the rotating speed of the rotating packed bed 4 to 1200r / min, the raw material toluene is cooled to -5°C to 0°C through the heat exchanger 41 before entering the rotating packed bed 4, and the flow rate of the toluene entering the system is 18 kg / h (the spray density is 9 meters 3 / m 2 .h), the flow rate of the mixed gas containing 6% sulfur trioxide (v / v) (the rest is dry air) is 30Nm 3 / h, after the two raw materials are rapidly mixed and reacted in the rotating packed bed 4, they enter the constant temperature reactor 38 through the rotating packed bed liquid outlet 18 and continue to complete the reaction (aging) at a suitable temperature (5-10) °C to obtain Product enters product storage tank 39 . The molar ratio of sulfur trioxide and toluene added to the reaction system is 0.41:1. The para-position content in the toluenesulfonic acid p...
Embodiment 2
[0039] Embodiment 2: the sulfonation of toluene
[0040] use figure 2 The plant scheme shown performs the sulfonation of toluene. Adjust the rotational speed of the rotary packed bed 4 to 1500 r / min. Before entering the rotary packed bed 4, the raw material toluene is cooled to 0°C-5°C through the heat exchanger 41, and the flow rate of the toluene entering the system is 18 kg / h, containing sulfur trioxide 8% (v / v) mixture (the rest is dry air) with a flow rate of 25 Nm 3 / h, after the two raw materials are quickly mixed and reacted in the rotating packed bed 4, the product enters the constant temperature reactor 38 through the liquid outlet 18 of the rotating packed bed and continues to complete the reaction (aging) at a suitable temperature (5-10) °C , get the product into the product storage tank 39. The molar ratio of sulfur trioxide and toluene added to the reaction system is 0.45:1. The para-position content in the toluenesulfonic acid produced by the reaction accou...
Embodiment 3
[0041] Embodiment 3: Sulfonation of toluene
[0042] use figure 2 The plant scheme shown performs the sulfonation of toluene. Adjust the rotational speed of the rotary packed bed 4 to 1800 r / min, and the raw material toluene is cooled to -5°C to 0°C through the heat exchanger 41 before entering the rotary packed bed 4, and the flow rate of the toluene entering the system is 18 kg / h, containing three The flow rate of sulfur oxide 10% (v / v) mixture (the rest is dry air) is 15 Nm 3 / h, after the two raw materials are rapidly mixed and reacted in the rotating packed bed 4, the product enters the constant temperature reactor 38 through the liquid outlet 18 of the rotating packed bed and continues to complete the reaction (aging) at a suitable temperature (5-10) °C. The resulting product enters product storage tank 39 . The molar ratio of sulfur trioxide and toluene added to the reaction system is 0.34:1. The para-position content in the toluenesulfonic acid produced by the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com