Application of vitamin K3 in preparation of drug for treating bacterial infection
A bacterial infection and vitamin technology, applied in the direction of antibacterial drugs, anhydride/acid/halide active ingredients, etc., can solve the problems such as no vitamin K antibacterial, no anti multidrug resistance bacteria and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
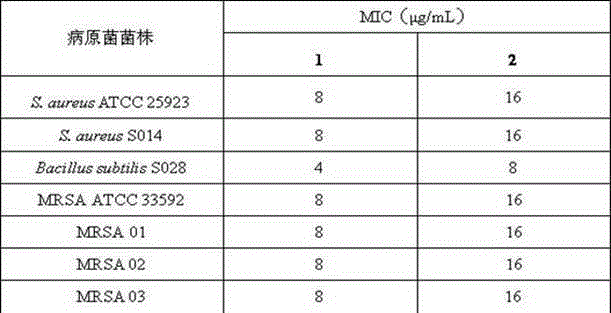
[0012] Vitamin K 3 antimicrobial and anti-multidrug-resistant bacteria test
[0013] 1. Experimental materials and methods
[0014] Test strain:
[0015] Staphylococcus aureus: Staphylococcus aureus ATCC 25923, S. aureus S014; Bacillus subtilis: Bacillus subtilis S028.
[0016] Methicillin-resistant Staphylococcus aureus standard strain: Methicillin-resistant S. aureus ATCC 33592 (MRSA ATCC 33592); Methicillin-resistant Staphylococcus aureus clinical strains: MRSA 01, MRSA 02 and MRSA 03.
[0017] MHB medium: 2.0 g beef extract powder, 1.5 g soluble starch, 17.5 g acid hydrolyzed casein, 1000 mL pure water, pH 7.4.
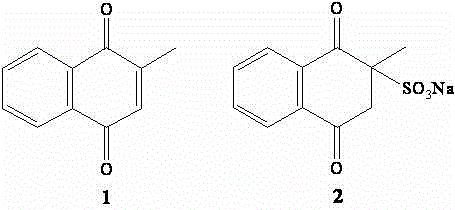
[0018] Preparation of test sample solution: Weigh each accurately 1 with 2 Appropriate amount, add a small amount of DMSO to dissolve, and then use MHB medium to prepare a test sample solution with a final concentration of 256 μg / mL (DMSO concentration is less than 5%).
[0019] According to the CLSI standard, the antibacterial and anti-multidrug...
Embodiment 2
[0027] Vitamin K 3 Tablet preparation
[0028] 1. Prescription Dosage per tablet 300 tablets
[0029] Vitamin K 3 (2-methyl-1,4-naphthoquinone, 1 ) 0.0750g 22.5g
[0030] Starch 0.0200g 6g
[0031] 10% starch slurry appropriate amount
[0032] Magnesium stearate appropriate amount;
[0033] 2. Preparation method:
[0034] (1) Preparation of 10% starch slurry: Add 2g of starch into about 20ml of purified water, heat and gelatinize to make 10% starch slurry.
[0035] (2) Granulation: Take the prescribed amount of 2-methyl-1,4-naphthoquinone ( 1 ) mixed with starch evenly, add an appropriate amount of 10% starch slurry to make soft material, granulate through a 16-mesh sieve, dry the wet granules at 40-50°C, granulate with a 16-mesh sieve and mix with magnesium stearate.
[0036] (3) Tablet compression: the above vitamin K 3 The granules are compressed on a tablet machine to obtain vitamin K 3 Tablets.
[0037] (4) Coating: vitamin K 3 Vegetarian tablets coated wi...
Embodiment 3
[0043] Vitamin K 3 Preparation of Injection
[0044]1. Prescription 500 sticks per stick
[0045] Vitamin K 3 (sodium bisulfite menadione, 2 ) 0.0200g 10.0g
[0046] Sodium bisulfite 0.0005g 0.25g
[0047] Add water for injection to 1000mL;
[0048] 2. Preparation method:
[0049] (1) Preparation of injection: Measure 80% of the prescribed amount of water for injection, saturate it with carbon dioxide, and add the prescribed amount of menadione sodium bisulfite ( 2 ) to make it dissolve, slowly add sodium bicarbonate step by step, stir to dissolve completely, then adjust the pH of the liquid to 2.5~3.5, and add water for injection saturated with carbon dioxide to a sufficient amount. with G 3 The vertical melting funnel pre-filtered, and then finely filtered with a 0.45 μm microporous membrane. Check filtrate clarity.
[0050] (2) Filling and fusion sealing: Immediately fill the filtered medicinal liquid into a 2 mL ampoule, and pass carbon dioxide into the upper s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com