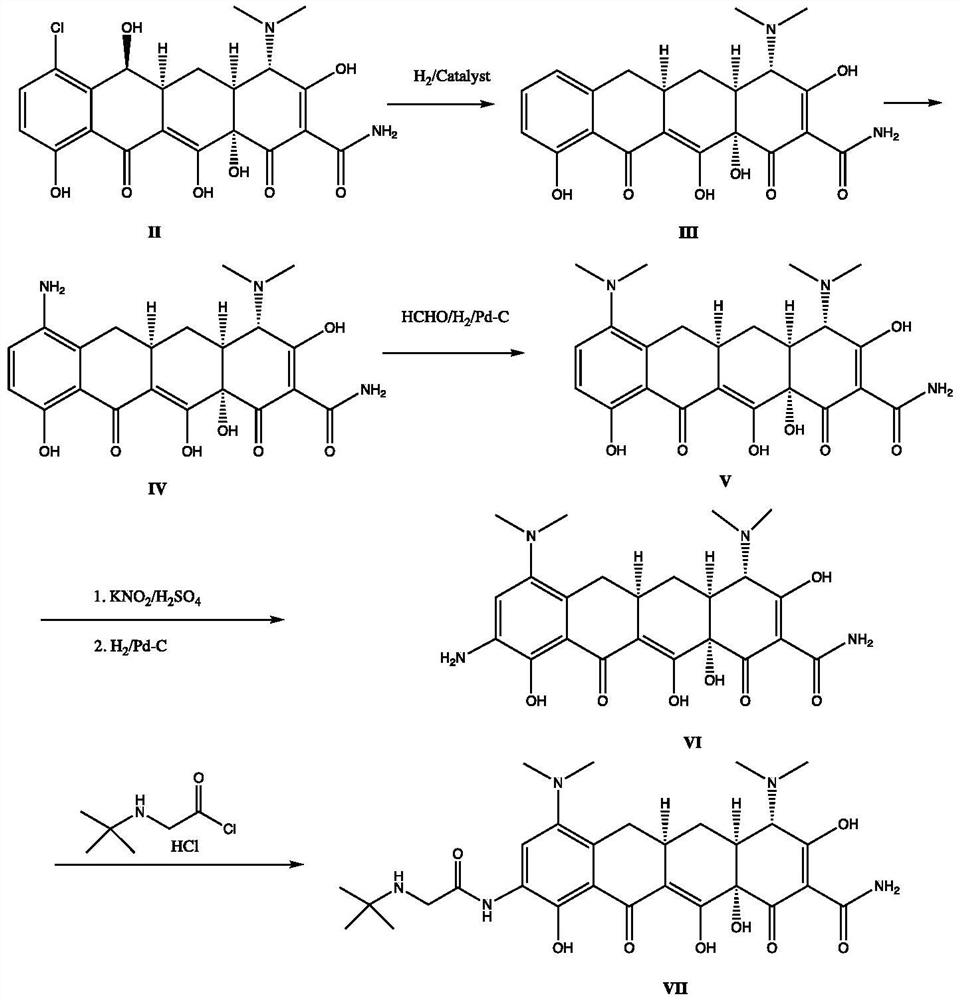
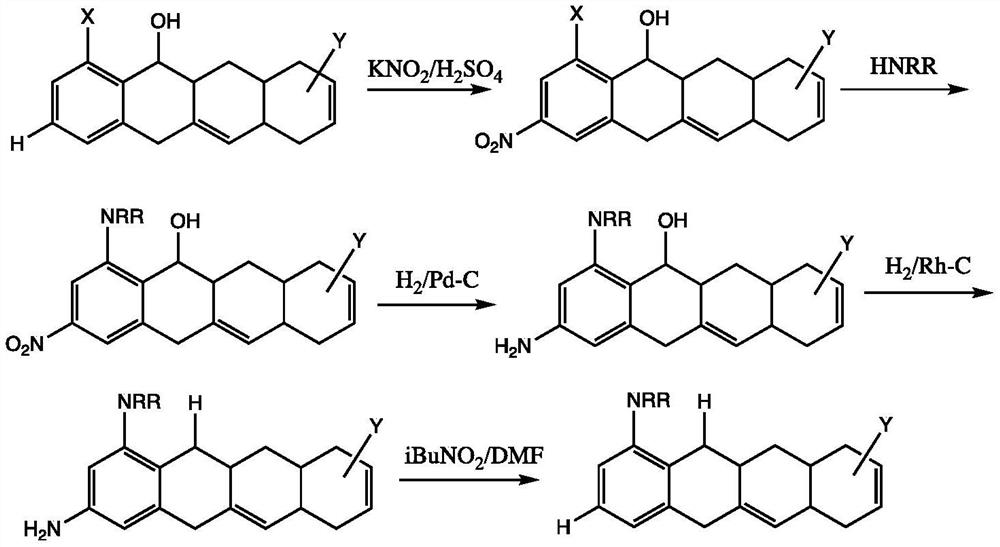
A kind of synthetic method of minocycline and its derivatives
A technology of aminominocycline and nitrominocycline, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the preparation of 9-nitrodemeclotetracycline
[0017] Demeclotetracycline hydrochloride (25.0 grams, 50 mmoles) was added in 180 milliliters of vitriol oil under the cooling of ice liquid, then control temperature of reaction, potassium nitrate (6.0 grams, 60 mmols) was added in batches, ice The mixture was stirred and reacted for 1 hour under liquid cooling, then poured into isopropyl ether cooled by ice liquid, a solid was precipitated, filtered, and the filter cake was washed with isopropyl ether to obtain 9-nitronoruretetracycline as a khaki solid. m / z: 509. 1 H NMR(DMSO-d6,500MHz)δ8.40(s,1H),4.75(s,1H),3.3(m,1H),3.23(m,6H),3.0-3.1(m,3H),3.0( m,3H), 2.59(m,2H), 2.4(m,2H), 1.7(m,1H).
Embodiment 2
[0018] Example 2: Synthesis of 6-hydroxyl-9-nitrominocycline
[0019] Add N,N-dimethylformamide (7 molar ratio), potassium hydroxide (2.5 molar ratio) and 9-nitronorautetracycline (10 mM Moore). After sealing, the reaction was stirred at 100°C for 24 hours. After cooling, the mixture was diluted with ethyl acetate. The organic layer was separated and the aqueous layer was extracted with ethyl acetate (3x100ml). The combined extracts were dried over anhydrous sodium sulfate, filtered, and the solvent was removed in vacuo. 6-Hydroxy-9-nitrominocycline is obtained. m / z: 518. 1 H NMR(DMSO-d6,500MHz)δ8.40(s,1H),4.75(s,1H),3.44(m,6H),3.3(m,1H),3.23(m,6H),3.0-3.1( m,3H), 3.0(m,3H), 2.59(m,2H), 2.4(m,2H), 1.7(m,1H).
Embodiment 3
[0020] Example 3: Synthesis of 6-hydroxyl-9-aminominocycline
[0021] 50 grams of urea was added to 200 milliliters of distilled water, stirred to dissolve it, 6-hydroxyl-9-nitrominocycline (26 grams, 50 mmoles) was added, and then the pH value was adjusted to 8.5 with sodium hydroxide solution, 6-Hydroxy-9-nitrominocycline is completely dissolved, the reaction solution is transferred to a 500ml autoclave, 0.4 g of 5% Pd / C catalyst is added, the mouth of the tank is sealed, and the air in the tank is replaced with nitrogen for three times, and finally Press hydrogen to tank pressure of 0.7 MPa, maintain this pressure by adjusting the hydrogen valve, and stir at room temperature for 6 hours. The gas in the tank was replaced with nitrogen three times, the reaction solution was poured out and the pH was adjusted to 1.0 with hydrochloric acid, and filtered. The filtrate was adjusted to pH=5.5 with sodium hydroxide solution. The obtained crystals were filtered to obtain a yellow pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com