Human immune globulin epsilon heavy chain constant region (hIgE Fc) fragment protein and preparation method and application thereof
A technology of human immunoglobulin and heavy chain constant region, applied in the field of biomedicine, can solve the problem of lack of effective drugs for the treatment of allergic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The present invention provides a kind of preparation method of human immunoglobulin epsilon heavy chain constant region (hIgE Fc) fragment protein, comprising the following steps:
[0087] (1) operably linking the nucleotide sequence encoding hIgE Fc to the expression vector to obtain a hIgE Fc recombinant expression vector;
[0088] (2) Transcribing the recombinant expression vector in step (1) into CHO cells, and screening to obtain engineered cell lines expressing hIgE Fc;
[0089] (3) cultivating the engineered cell strain obtained in step (2) under suitable conditions;
[0090] (4) Isolating the human immunoglobulin ε heavy chain constant region (hIgE Fc) fragment protein from the culture product in step (3).
[0091] The step of cultivating the engineered cell strain expressing hIgE Fc protein under suitable conditions in step (3) comprises: cultivating the engineered cell strain obtained through screening in a medium that does not contain bovine serum, and the c...
Embodiment 1
[0100] Expression of embodiment 1 hIgE Fc protein
[0101] The expression process of hIgE Fc protein includes the following steps: synthesizing the gene; inserting the gene into the expression vector, transfecting CHO cells with the expression vector; using Hygromycin to screen the CHO engineered cell line expressing the hIgE Fc fusion protein, cultivating the cell line, and finally extracting from the cell culture medium Separation and purification of RANKL-Fc protein. specifically:
[0102] 1) Gene synthesis
[0103] Entrust a professional company for gene synthesis to synthesize the nucleotide sequence shown in SEQ ID NO: 2, and add a BamHI restriction site and a Kozak sequence at the 5' end; add an Xba I restriction site at the 3' end .
[0104] 2) Construction of expression vector
[0105] After obtaining the above-mentioned synthesized gene from Gene Synthesis Company, use BamHI and Xba I to double-enzyme digest the gene and vector pCDNA3.1, recover the digested fra...
Embodiment 2hI
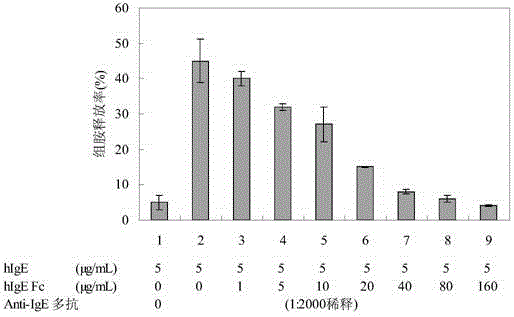
[0110] Example 2 hIgE Fc protein inhibits activation of basophils to release histamine
[0111] Basophils were isolated from the peripheral blood of healthy people, distributed into 96 plates according to 1×106cells / well, and then added Figure 4 The amount of hIgE and hIgE Fc shown in , after 4 hours, carefully aspirate the culture supernatant, and wash twice with calcium and magnesium-free HBSS buffer, anti-stimulate with 100 μL Anti-IgE Fc, after half an hour, remove the supernatant , using histamine ELISA assay kit to detect the concentration of histamine. The percentage of histamine released is the ratio of the amount of histamine released after the cells were stimulated to the amount of histamine released after the same amount of cells were boiled at 100°C for 6 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com