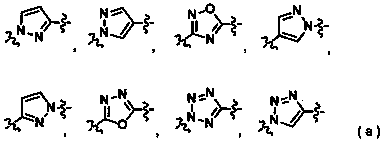
Branched chain alkyl heteroaromatic ring derivative
An alkyl and alkoxy technology, which is applied in drug combination, digestive system, endocrine system diseases, etc., can solve the problem of not disclosing OX receptor antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1003] Example 1: N-ethyl-N-{(2S)-1-[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]prop-2-yl}-5 -Methyl-2-(2H-1,2,3-triazol-2-yl)benzamide
[1004] [Formula 134]
[1005]
[1006] To (2S)-N-ethyl-1-[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]propan-2-amine disalt obtained in Reference Example 8 5-Methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid (0.11 g, 0.52 mmol) was added to a DMF (5.0 mL) solution of acid salt (0.14 g, 0.44 mmol) , HATU (0.25 g, 0.65 mmol) and DIPEA (0.68 mL, 3.92 mmol), and the resulting mixture was stirred at room temperature for 24 hours. Water was added to the reaction solution, followed by extraction with EtOAc. The organic layer was saturated NaHCO 3 aqueous and brine washes and MgSO 4 After drying, the desiccant was filtered off, and the solvent was distilled off under reduced pressure. The obtained residue was purified by column chromatography (KP-OH 25 g, hexane / EtOAc = 80 / 20-20 / 80) and HPLC to give the title compound (0.027 g) (colorless and amorpho...
Embodiment 18
[1014] Example 18: N-ethyl-N-{(2S)-1-[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]propan-2-yl}-2 -(2H-1,2,3-triazol-2-yl)benzamide
[1015] [Formula 135]
[1016]
[1017] To N-{(2S)-1-[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]propan-2-yl}-2-( 2H-1,2,3-triazol-2-yl)benzamide (0.13 g, 0.33 mmol) in DMF (5.0 mL) was added 60% NaH (0.020 g, 0.50 mmol), and the resulting mixture Stir at room temperature for 30 minutes. EtI (0.032 mL, 0.40 mmol) was added to the reaction solution, and the resulting mixture was stirred at room temperature for 24 hrs. Water was added to the reaction solution, followed by extraction with EtOAc. The organic layer was saturated NaHCO 3 aqueous and brine washes and MgSO 4 After drying, the desiccant was filtered off, and the solvent was distilled off under reduced pressure. The obtained residue was purified by column chromatography (KP-NH 28 g, hexane / EtOAc = 80 / 20-40 / 60) to obtain the title compound (0.058 g) (colorless and amorphous).
[1018] ...
Embodiment 34
[1026] Example 34: N-Ethyl-5-fluoro-N-{(2S)-1-[4-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]propan-2- Base}-2-(pyrimidin-2-yl)benzamide
[1027] [Formula 136]
[1028]
[1029] To 5-fluoro-N-{(2S)-1-[4-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]propan-2-yl obtained in Reference Example 81 }-2-(pyrimidin-2-yl)benzamide (0.028 g, 0.067 mmol) in DMF (1.5 mL) was added 60% NaH (0.0039 g, 0.10 mmol), and the resulting mixture was stirred at room temperature 10 minutes. EtI (0.0064 ml, 0.080 mmol) was added to the reaction solution, and the resulting mixture was stirred at room temperature for 1.5 hr. 60% NaH (0.0039 g, 0.10 mmol) was added to the reaction solution, the mixture was stirred at room temperature for 10 minutes, then EtI (0.0064 ml, 0.080 mmol) was added thereto, and the resulting mixture was stirred at room temperature for 16 Hour. will saturate NH 4 Aqueous Cl solution was added to the reaction solution, followed by extraction with chloroform. The organic layer was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com