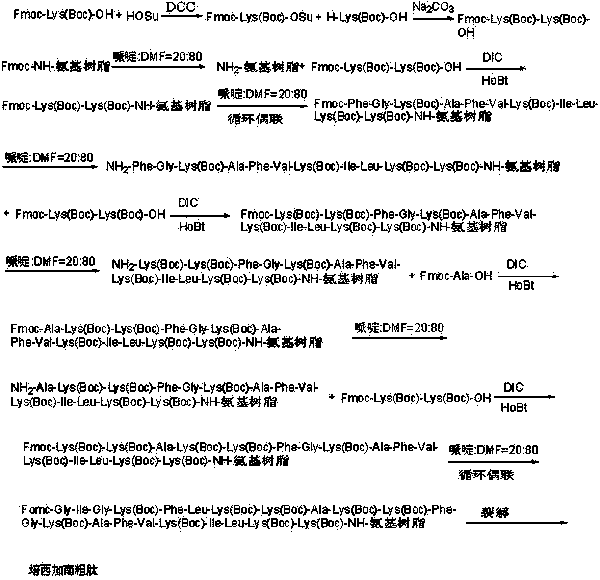
Preparation method of pexiganan
A liquid phase method and peptide fragment technology, which is applied in the field of preparation of polypeptide antibiotics, can solve the problems of unsuitability for large-scale production, low purity and yield, and long synthesis cycle, and achieve low cost, low cost, and short synthesis cycle. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Synthesis of Fmoc-Lys(Boc)-OSu Activated Ester
[0065] Weigh 468.50g Fmoc-Lys(Boc)-OH (1.0mol), add 138.10g HOSu (1.2mol) into 2000ml DMF, add 247.59g DCC (1.2mol) under ice-water bath, react for 1 hour, heat up to room temperature for reaction 3 After 1 hour, the reaction solution was filtered, the mother liquor was spin-dried, dissolved in DCM, filtered, recrystallized from ice ethanol 3 times, filtered, and dried by a solid oil pump to obtain 503.37g of Fmoc-Lys(Boc)-OSu activated ester with a yield of 89%.
Embodiment 2
[0066] Example 2: Synthesis of Fmoc-Lys(Boc)-Lys(Boc)-OH
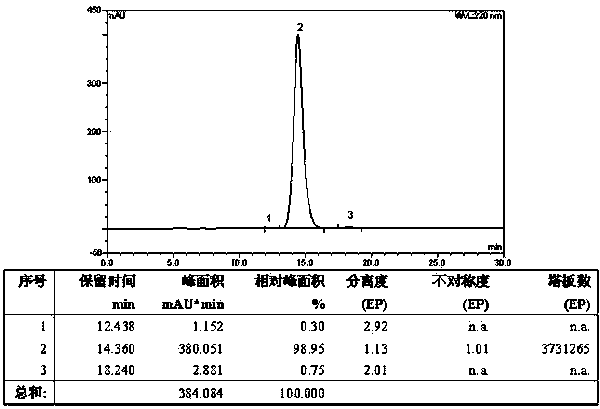
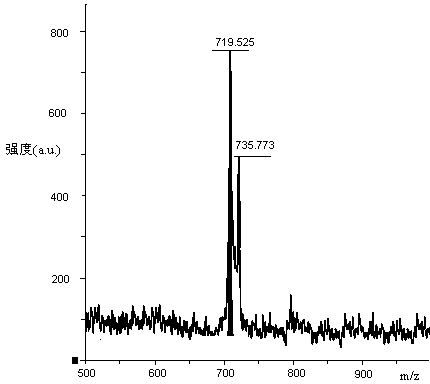
[0067] Weigh 123.15g H-Lys(Boc)-OH (0.5mol), 282.79g Fmoc-Lys(Boc)-OSu (0.5mol) and 79.50g Na 2 CO 3 (0.75mol) was added to a mixed solution of 500ml water and 500ml THF to dissolve, react overnight at room temperature, adjust the pH to 7 with 10% dilute hydrochloric acid, remove THF by rotary evaporation, and then adjust the pH to 3. A large white precipitate was obtained which was filtered. The obtained white precipitate is recrystallized with glacial ethanol, and the obtained solid oil pump is dried to obtain 296.15g of Fmoc-Lys(Boc)-Lys(Boc)-OH and its HPLC spectrogram is as follows: figure 2 Shown, HPLC purity is 98.95%, yield 85%; Its mass spectrum is as image 3 Shown, [M+Na] + : 719.525, [M+K] + : 735.773, the theoretical exact molecular weight of the dipeptide fragment Fmoc-Lys(Boc)-Lys(Boc)-OH is: 696.37, the mass spectrometry result of the sample is consistent with the theoretical molecular weight, and...
Embodiment 3
[0068] Embodiment three: adopting degree of substitution is the synthesis of the Rink Amide AM resin of 0.10mmol / g to Fmoc-Lys(Boc)-Lys(Boc)-Rink Amide AM resin
[0069] Weigh 100.00 g of Rink amide AM resin (10 mmol) with a substitution degree of 0.10 mmol / g, add it to a solid-phase reaction column, wash it once with DMF, and swell the resin with DCM for 30 minutes, and use a volume ratio of 1:4 The deprotection solution composed of piperidine and DMF was reacted for 5 minutes, washed once with DMF, reacted for 10 minutes with the deprotection solution composed of piperidine and DMF with a volume ratio of 1:4, washed 6 times with DMF, and weighed 20.91g Fmoc -Lys(Boc)-Lys(Boc)-OH (30mmol) and 4.05g HOBt (30mmol) were added to the mixed solution of DCM and DMF with a volume ratio of 1:1, and 3.79g DIC (30mmol) was added under ice-water bath for activation and then added to the above In the reaction column that resin is housed, react for 2 hours, wash 3 times with DMF, DCM wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com