Rheum emodin single-chain biquaternary ammonium salt with antitumor activity and preparation method thereof
A technology of double quaternary ammonium salt and anticancer activity, which is applied in the field of emodin single chain double quaternary ammonium salt with anticancer activity and its preparation, which can solve the problem of insufficient anticancer activity, lack of direct medicine, poor water solubility, etc. problems, to achieve good application prospects and good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
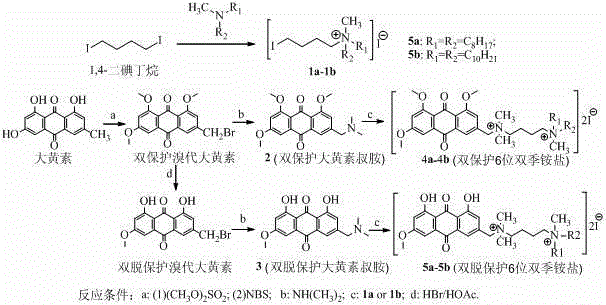
[0021] Example 1: Synthesis of iodobutyl quaternary ammonium salt
[0022] Take 0.45 mL of methyl bisoctyl tertiary amine or methyl bisdecyl tertiary amine, place it in a 50 mL three-necked flask, add 15 mL of ethylene glycol methyl ether, heat and stir at 100°C, slowly add 0.25 mL of 1,4-diiodo Butane, react for 12 hours and then cool to room temperature. After the solvent is removed by rotary evaporation, the obtained solid is subjected to silica gel column chromatography using dichloromethane-acetone as eluent, and the volume ratio is 45:1→30:1→15:1→ 10:1 gradient elution to obtain iodobutyl quaternary ammonium salt 1a or 1b The characterization data are as follows:
[0023] N-(4-iodobutyl)-N-methyl-N-octyloctane-1-ammonium iodide ( 1a ): Red-brown solid, yield 75%. 1 H-NMR (400MHz, CDCl 3 ) δ: 3.58 (t, J =5.6Hz, 2H, C H 2 I), 3.43 (t, J =1.6Hz, 2H, N + C H 2 (CH 2 ) 3 I), 3.39 (s, 3H, N + C H 3 ), 3.34 (t, J =5.6Hz, 4.0H, 2×N + C H 2 (CH 2 ) 8 CH 3 ), 1.95 (m, 4H, (C H 2 ) 2 CH...
Embodiment 2
[0025] Example 2: Synthesis of double-protected bromo-emodin
[0026] Dissolve 1.6g (5.9mmol) of emodin in 200ml of acetone, add 10g (73mmol) of anhydrous potassium carbonate, slowly add 4ml (43mmol) of dimethyl sulfate under reflux, and react under reflux for 24 hours. Cool to room temperature and After removing part of the solvent by rotary evaporation, add 80ml of water and stir for 30min, then filter with suction. Wash the filter cake with acetone to obtain 1.34g of bright yellow solid trimethylemodin; dissolve 0.8g (2.56mmol) of trimethylemodin in 60ml CCl 4 , Add 0.20g initiator benzoyl peroxide (BPO) and 1.6g (9.0mmol) brominated reagent N-bromosuccinimide (NBS), reflux reaction in a three-necked flask for 25 h, and cool to room temperature to obtain Yellow solid, use a small amount of CCl 4 After washing with acetone, water and acetone, it was subjected to silica gel column chromatography and separated with dichloromethane as the eluent to obtain 0.83 g of yellow solid di-...
Embodiment 3
[0028] Example 3: Synthesis of double-protected emodin tertiary amine
[0029] 100mg (0.26mmol) of the double-protected bromo-emodin synthesized in Example 2 was dissolved in chloroform, poured into a 50mL three-necked flask, and 140mg (2.5mmol) KOH, 5mL water, 50mg TEBA (benzyl triethyl chloride) Ammonium chloride, as a phase transfer catalyst), and 0.35 mL of 33% mass concentration of dimethylamine in water (2.6 mmol), stirred at room temperature for 4 hours, after the reaction, the solution was extracted with 15 mL of chloroform three times, combined the organic layers, and rotary evaporated After removing the solvent, the obtained solid is subjected to silica gel column chromatography with dichloromethane-acetone as the eluent, and the volume ratio is 45:1→30:1→15:1→10:1→5:1→2:1 Gradient elution to obtain double-protected emodin tertiary amine 2 (64.7mg, 0.18mmol), the product characterization data are as follows:
[0030] 3-[(Dimethylamino)methyl]-1,6,8-trimethoxy-9,10-anthr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com