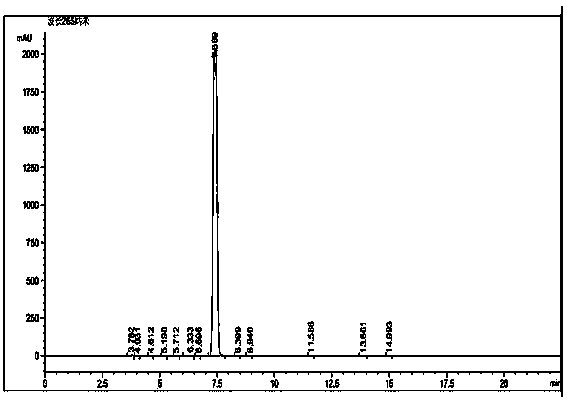
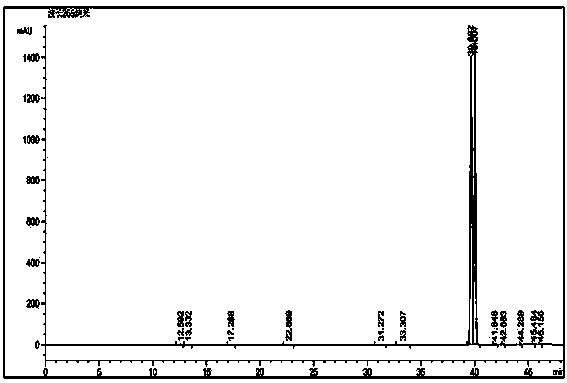
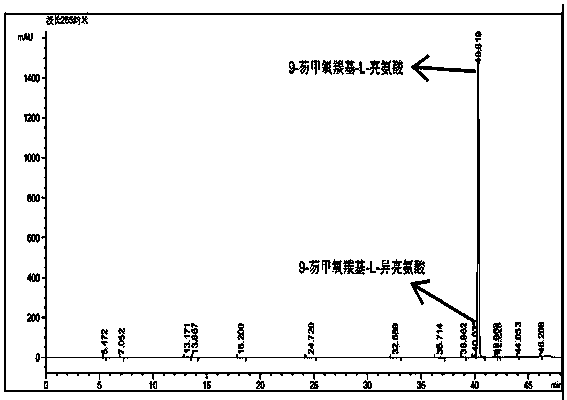
Method for reversed phase separation of derived leucine and isoleucine
A technology of isoleucine and leucine, applied in material separation, analysis materials, measuring devices, etc., can solve the problems of high equipment requirements, high cost, difficult separation of derivatized amino acids, etc., achieve good economic value, increase equipment cost, good reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] Below in conjunction with embodiment further illustrate the present invention.
[0033] 1. Mobile phase preparation: 0.1% trifluoroacetic acid aqueous solution
[0034] Measure 400 ml of deionized water into the solvent bottle with a graduated cylinder, then accurately measure 0.4 ml of analytically pure trifluoroacetic acid into 400 ml of deionized water with a pipette, and put the solvent bottle into ultrasonic vibration for later use.
[0035] 0.1% trifluoroacetic acid acetonitrile solution:
[0036] Measure 400 ml of chromatographic grade acetonitrile into the solvent bottle with a graduated cylinder, then accurately measure 0.4 ml of analytically pure trifluoroacetic acid into 400 ml of acetonitrile with a pipette, and put the solvent bottle into ultrasonic vibration for later use.
[0037] 2. Preparation of sample solution
[0038] Preparation of a single sample:
[0039] Weigh 5 mg of N-(9-fluorenylmethoxycarbonyl)-L-leucine and 5 mg of N-(9-fluorenylmethoxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com