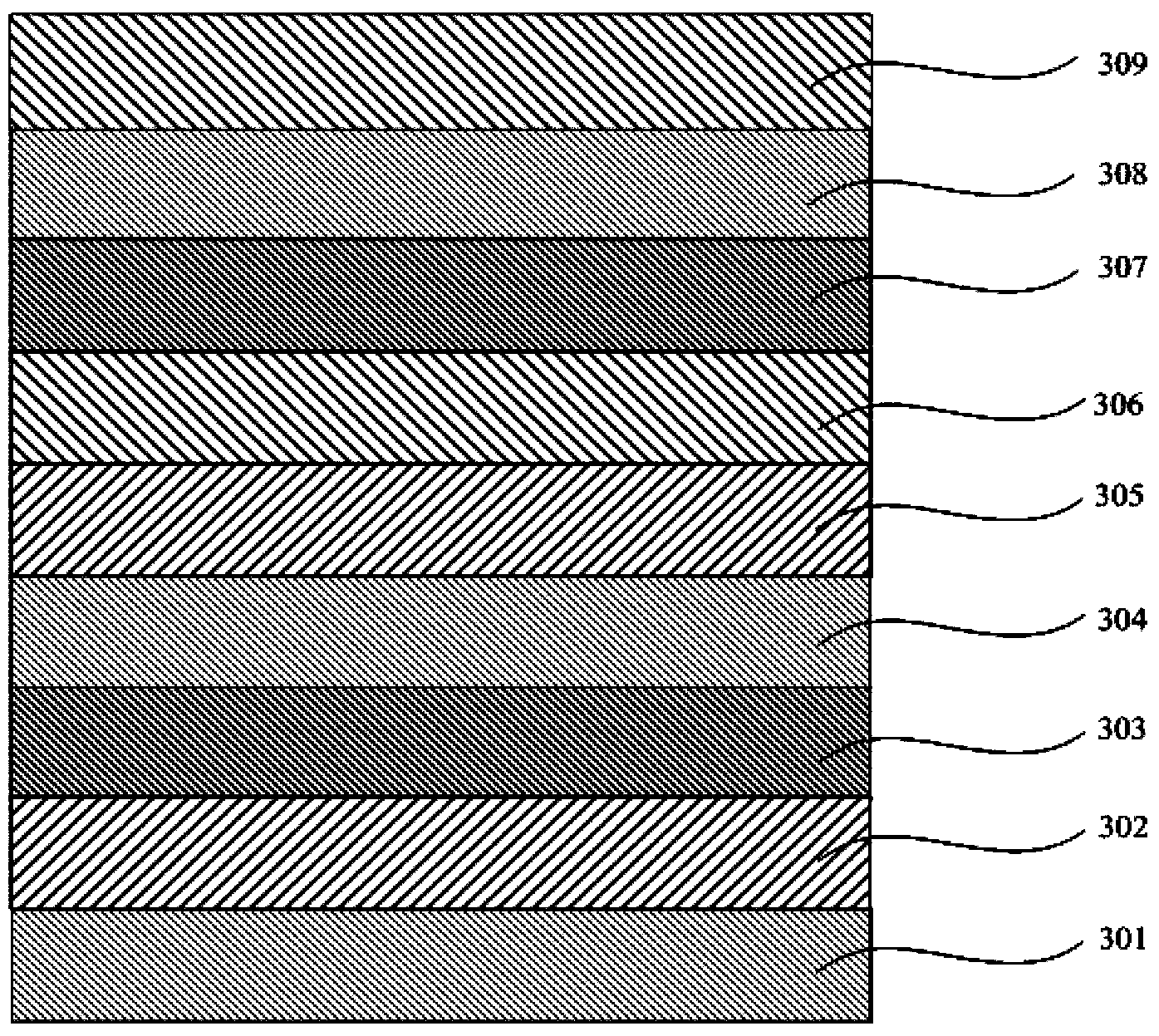
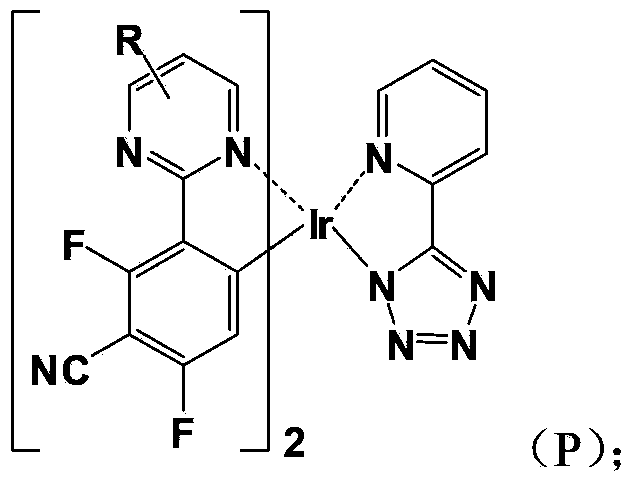
Organic electro-phosphorescent material and preparation method thereof, and organic electroluminescent device
A phosphorescent material, electromechanical technology, applied in the field of phosphorescent materials, can solve the problems of blue light phosphorescent material luminescence color purity, luminous efficiency device efficiency attenuation bottleneck, etc., to reduce self-quenching phenomenon, improve LUMO energy level, and increase the effect of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation process of the organic electrophosphorescent material (P) of the present invention is roughly divided into the following steps:
[0039] (1) Synthesize compound C through Suzuki coupling reaction of compound E and compound F; wherein, compound F is 2,4-difluoro-3-cyanophenylboronic acid, and the structural formulas of compound E and compound C are as follows:
[0040] Compound E is Compound C is Among them, R is C 1 ~C 20 straight-chain or branched-chain alkyl groups, or hydrogen atoms;
[0041] (2) Reaction of compound C prepared in step (1) with compound D to generate a chlorine-bridged dimer, namely compound A. Wherein, compound D is trihydrate iridium trichloride IrCl 3 ·3H 2 O. The structural formula of compound A is as follows:
[0042]
[0043] (3) Compound A prepared in step (2) is used as the main structure of the ring metal ligand, and 5-(2'-pyridyl)-1,2,3,4-tetrazole (compound B) is used as An auxiliary ligand source is used to r...
Embodiment 1
[0049] The organic electrophosphorescent material (P) disclosed in this example is the complex bis(2-(4′,6′-difluoro-5′-cyanophenyl)pyrimidine-N,C2′)(5-( 2'-pyridyl)-1,2,3,4-tetrazole) iridium, its structural formula is as follows:
[0050]
[0051] It is prepared by the following steps:
[0052] (1) Synthesis of 2-(2′,4′-difluoro-3′-cyanophenyl)pyrimidine
[0053] The reaction formula for the synthesis of 2-(2′,4′-difluoro-3′-cyanophenyl)pyrimidine is as follows:
[0054]
[0055] The specific steps are: under the protection of nitrogen, mix 1.59g (10mmol) 2-bromopyrimidine, 2.20g (12mmol) 2,4-difluoro-3-cyanophenylboronic acid and 0.58g (0.5mmol) tetrakis (triphenyl Phosphorus) palladium was dissolved in 40mL of toluene, and then 20mL of an aqueous solution containing 2.76g (20mmol) of potassium carbonate was added dropwise to the reaction system. Heat to 100°C and stir the reaction for 6h. After the reaction solution was cooled to room temperature, it was extracte...
Embodiment 2
[0070] The organic electrophosphorescent material (P) disclosed in this example is the complex bis(2-(4',6'-difluoro-5'-cyanophenyl)-5-methylpyrimidine-N,C 2 ')(5-(2'-pyridyl)-1,2,3,4-tetrazole) iridium, its structural formula is as follows:
[0071]
[0072] It is prepared by the following steps:
[0073] (1) Synthesis of 2-(2′,4′-difluoro-3′-cyanophenyl)-5-methylpyrimidine
[0074] The reaction formula for the synthesis of 2-(2′,4′-difluoro-3′-cyanophenyl)-5-methylpyrimidine is as follows:
[0075]
[0076] The specific steps are: under nitrogen protection, mix 1.73g (10mmol) 2-bromo-5-methylpyrimidine, 1.83g (10mmol) 2,4-difluoro-3-cyanophenylboronic acid and 0.28g (0.4mmol) Dichlorobis(triphenylphosphine)palladium was dissolved in 50 mL of N,N-dimethylformamide solution (ie DMF), and then 25 mL of an aqueous solution containing 3.18 g (30 mmol) of sodium carbonate was added dropwise to the reaction system. Heated to 90°C and stirred for 8h. After the reaction sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com