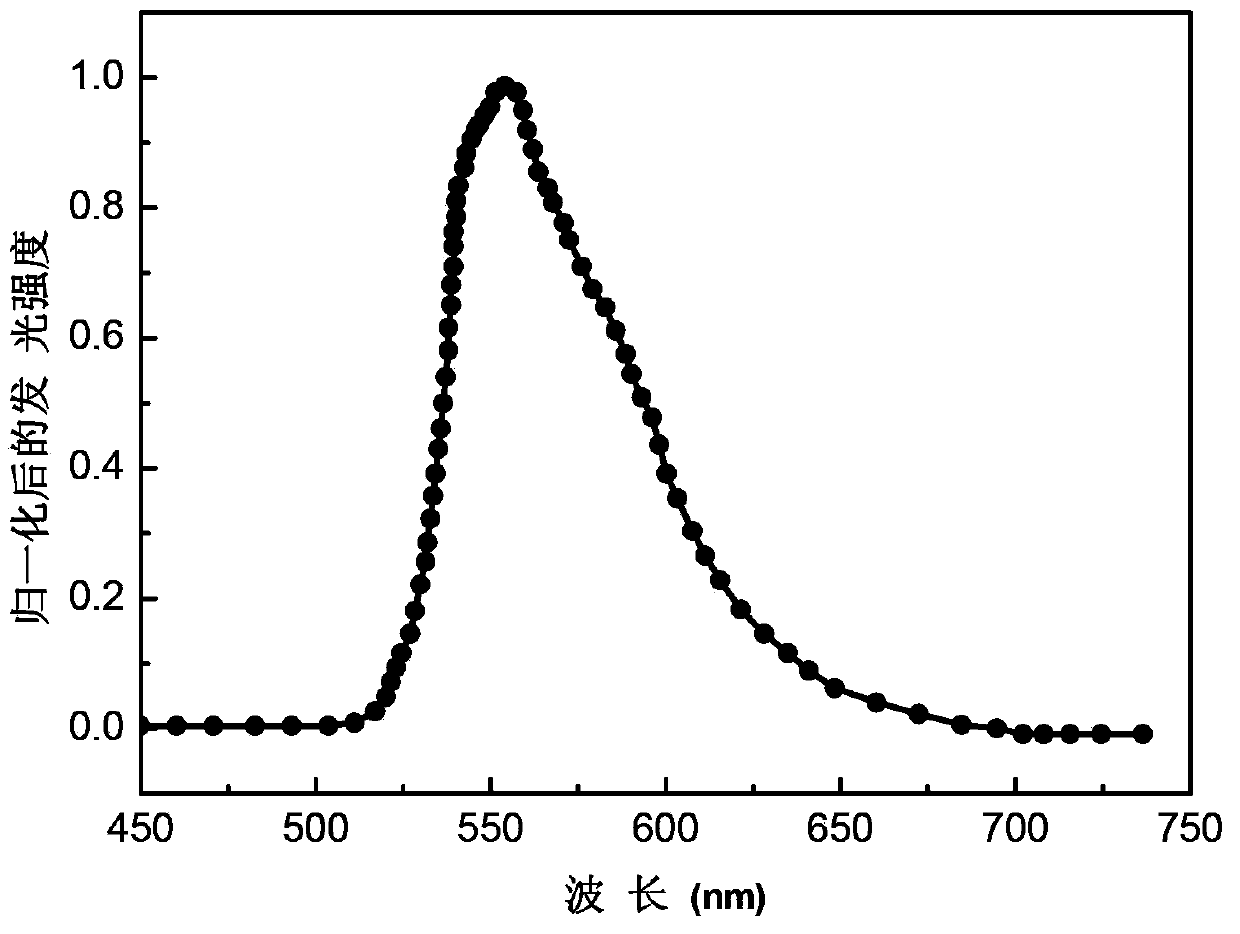
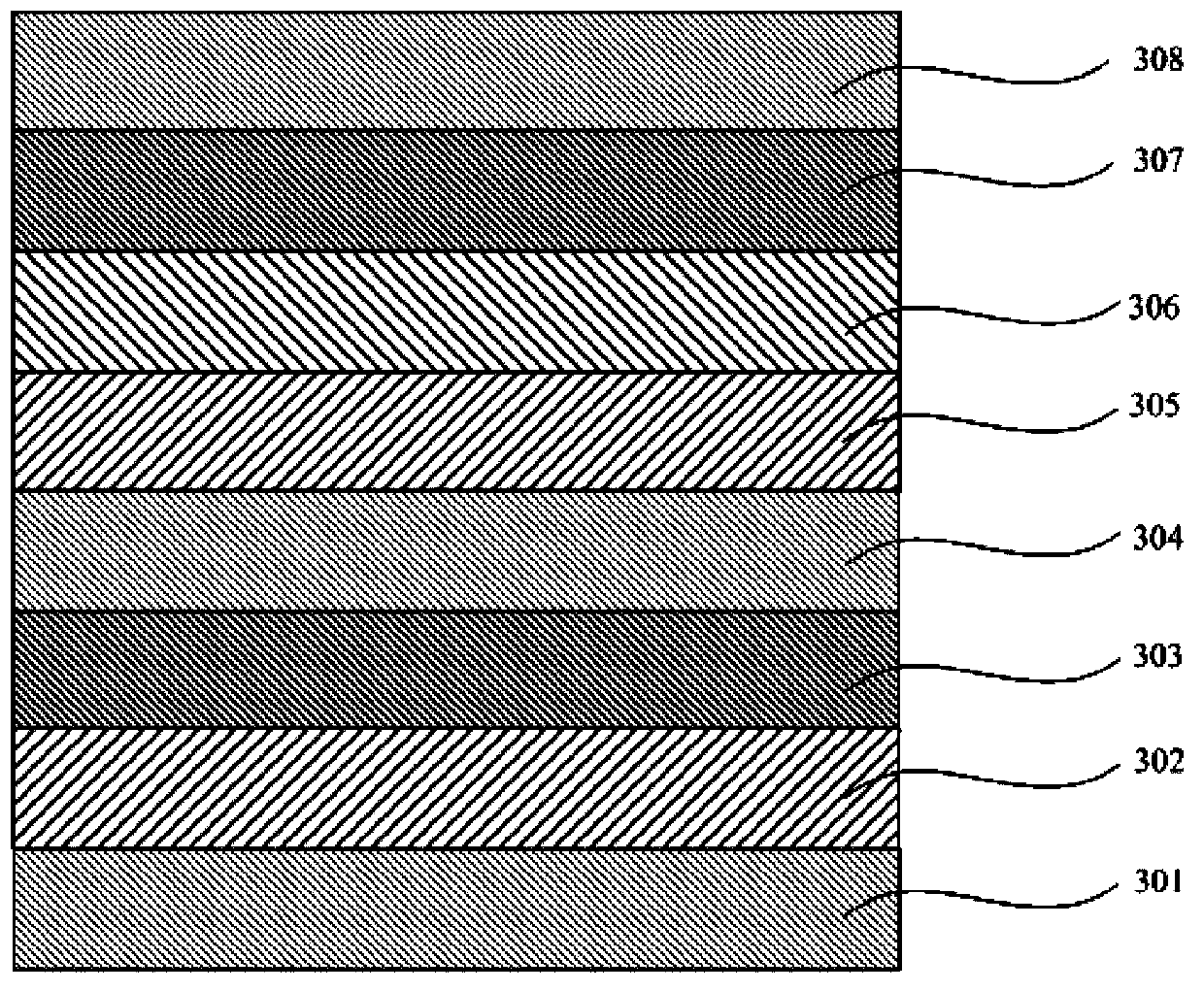
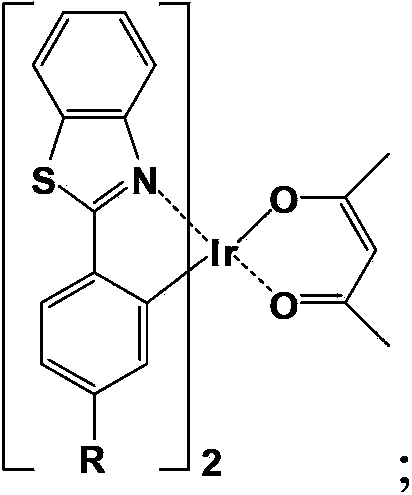
Green-yellow light-emitting organic electroluminescent material, its preparation method and organic electroluminescent device
A kind of electroluminescence, yellow-green light technology, applied in the field of yellow-green light organic electroluminescence material and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the above-mentioned yellow-green light organic electroluminescent material provided by the present invention comprises the following steps:
[0040] S1. Under the protection of inert gas (at least one of nitrogen and argon, the same below), the structural formula is Compound A (2-bromobenzothiophene), the structural formula is Dissolve the compound B (4-alkylphenylboronic acid or 4-alkoxyphenylboronic acid) and the rake catalyst in the solvent, then add sodium carbonate or potassium carbonate to the solvent, heat up to reflux state, and stir for 6~15h. Cool naturally to room temperature, separate and purify the reaction solution, and obtain the structural formula: Compound C (2-(4'-alkylphenyl)benzothiazole or 2-(4'-alkoxyphenyl)benzothiazole); wherein, the molar ratio of compound A to compound B is 1:1.2 The molar amount of sodium carbonate or potassium carbonate is 3 times that of compound A; the solvent is a mixed solvent composed of to...
Embodiment 1
[0060] Example 1: Complex bis(2-(4'-methylphenyl)benzothiazole-N,C 2 ') (acetylacetonate) iridium synthesis
[0061] (1) Synthesis of 2-(4'-methylphenyl)benzothiazole
[0062] Under nitrogen protection, (2.14g, 0.01mol) 2-bromobenzothiophene, (1.63g, 0.012mol) 4-tolueneboronic acid and (0.35g, 0.0003mol) Pd (PPh 3 ) 4 Dissolve in a mixed solution consisting of 15mL of toluene and 5ml of ethanol, add 15mL of 2M Na 2 CO 3 Aqueous solution, heated to reflux state, stirring reaction 10h. After naturally cooling to room temperature, an appropriate amount of distilled water was added and extracted with toluene. After combining the organic phases, washing with water, anhydrous MgSO 4 The organic phase is dried. After filtration, the filtrate was evaporated to remove the solvent under reduced pressure, and the mixture of n-hexane and dichloromethane with a volume ratio of 1:1 was used as the eluent for silica gel column chromatography to obtain 1.58 g of solid, with a yield of ...
Embodiment 2
[0084] Example 2: Complex bis(2-(4'-ethylphenyl)benzothiazole-N,C 2 ') (acetylacetonate) iridium synthesis
[0085] (1) Synthesis of 2-(4'-ethylphenyl)benzothiazole
[0086] Under nitrogen protection, (2.14g, 0.01mol) 2-bromobenzothiophene, (1.80g, 0.012mol) 4-ethylphenylboronic acid and (0.35g, 0.0003mol) Pd (PPh 3 ) 4Dissolve in 20mL THF, add 15mL of 2M Na 2 CO 3 Aqueous solution, heated to reflux state, stirring reaction for 15h. After naturally cooling to room temperature, an appropriate amount of distilled water was added and extracted with toluene. After combining the organic phases, washing with water, anhydrous MgSO 4 The organic phase is dried. After filtration, the filtrate was evaporated to remove the solvent under reduced pressure, and the mixture of n-hexane and dichloromethane with a volume ratio of 2:1 was used as the eluent for silica gel column chromatography to obtain 1.44 g of solid, with a yield of 60.2%. The reaction equation is as follows:
[008...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com