Method for preparing high-purity lithium carbonate with co-production of lithium fluoride by employing crude lithium carbonate
A technology of high-purity lithium carbonate and lithium carbonate, applied in the direction of lithium carbonate;/acid carbonate, lithium halide, products, etc., can solve the problems that the products cannot meet the requirements of high-purity lithium carbonate, and achieve social benefits Significant environmental and environmental benefits, significant environmental benefits and economic benefits, solving the problem of single raw materials for preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
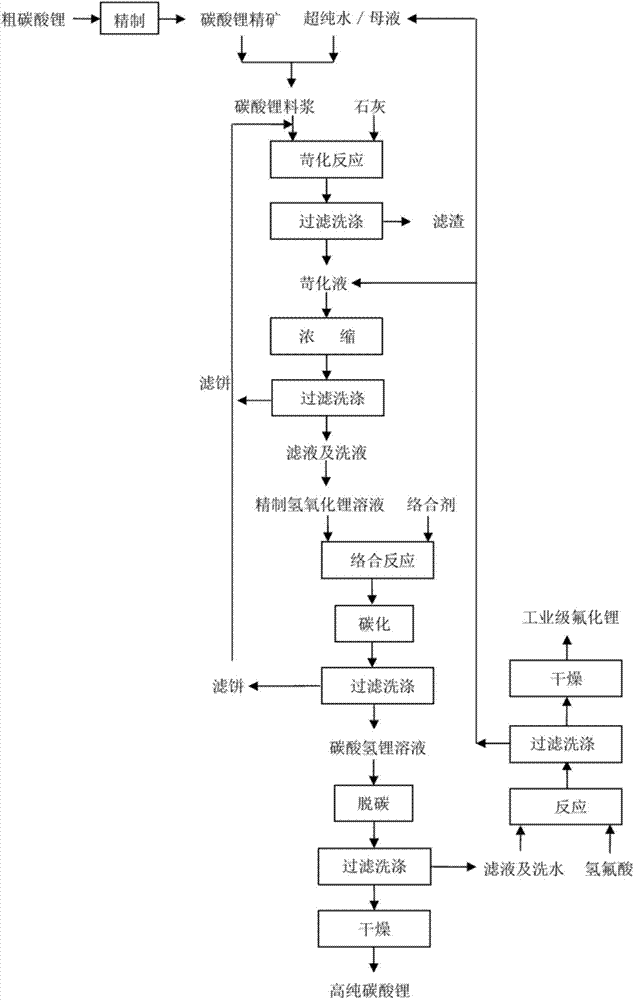
[0047] Utilize crude lithium carbonate of the present embodiment to prepare the method for high-purity lithium carbonate co-production lithium fluoride, as figure 1 shown, including the following steps:
[0048] 1) Refining of crude lithium carbonate: take 3522 kg of crude lithium carbonate (mass content of lithium carbonate is 40%) from salt lake, add water to prepare slurry A, the liquid-solid ratio (l / s) is 1.5, stir at 100°C for 0.5h, Dissolve soluble carbonate compounds such as Na and K, filter and wash after the dissolution is complete, and obtain 2296kg of lithium carbonate concentrate; the washing water is used to produce other products, such as potassium fluoride, sodium fluoride, etc.;
[0049] 2) Preparation of crude lithium hydroxide solution: add water to the lithium carbonate concentrate obtained in step 1) to prepare lithium carbonate slurry, add lime and carry out causticization reaction, the mol ratio of carbonate in lime and lithium carbonate slurry is 1.0:1 ...
Embodiment 2
[0055] Utilize crude lithium carbonate of the present embodiment to prepare the method for high-purity lithium carbonate co-production lithium fluoride, as figure 1 shown, including the following steps:
[0056] 1) Refining of crude lithium carbonate: take 2348kg of crude lithium carbonate (mass content of lithium carbonate is 60%) from salt lake, add water to prepare slurry A, the liquid-solid ratio (l / s) is 1.0, stir at 20°C for 10h, dissolve Soluble carbonate compounds such as Na and K are filtered and washed after being completely dissolved to obtain 2000kg of lithium carbonate concentrate; the washing water is used to produce other products, such as potassium fluoride, sodium fluoride, etc.;
[0057] 2) Preparation of crude lithium hydroxide solution: adding water to the lithium carbonate concentrate obtained in step 1) is mixed with lithium carbonate slurry, adding lime to carry out causticization reaction, and the mol ratio of carbonate in lime and lithium carbonate slu...
Embodiment 3
[0063] Utilize crude lithium carbonate of the present embodiment to prepare the method for high-purity lithium carbonate co-production lithium fluoride, as figure 1 shown, including the following steps:
[0064] 1) Refining of crude lithium carbonate: take 2013kg of crude lithium carbonate (mass content of lithium carbonate is 70%) from salt lake, add water to prepare slurry A, the liquid-solid ratio (l / s) is 1.2, stir at 80°C for 3h, dissolve Soluble carbonate compounds such as Na and K are filtered and washed after being completely dissolved to obtain 1899kg of lithium carbonate concentrate; the washing water is used to produce other products, such as potassium fluoride and sodium fluoride;
[0065] 2) Preparation of crude lithium hydroxide solution: adding water to the lithium carbonate concentrate obtained in step 1) is mixed with lithium carbonate slurry, adding lime to carry out causticization reaction, and the mol ratio of carbonate in lime and lithium carbonate slurry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com