Macromolecular hindered phenol antioxidant containing thioether and carbamate group and preparation method and application thereof
A technology of urethane-based and hindered phenol antioxidants, which is applied in the field of macromolecular hindered phenol antioxidants and its preparation, can solve the problems of large addition amount and low content of antioxidant groups, and achieve small addition amount, Effect of high molecular weight and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Synthesis of hindered phenol intermediates containing alcoholic hydroxyl groups and thioether groups: Dissolve 0.02mol of antioxidant GM and 0.04mol of 2-mercaptoethanol (ME) in a 150mL three-neck flask equipped with magnetic stirring and condenser Obtain reactant toluene solution in 50g toluene; Dissolve 0.80mmol triethylamine in 10g toluene to obtain triethylamine toluene solution. At 50°C, the triethylamine toluene solution was added dropwise to the reactant toluene solution within 30 min. After constant temperature reaction for 3 hours, it was transferred to a round-bottomed flask, and toluene and excess 2-mercaptoethanol were distilled off under reduced pressure at 100°C to obtain a hindered phenol intermediate GM-ME containing alcoholic hydroxyl groups and thioether groups. The molecular structure of antioxidant GM and hindered phenol intermediate GM-ME containing alcoholic hydroxyl group and thioether group is as follows:
[0033]
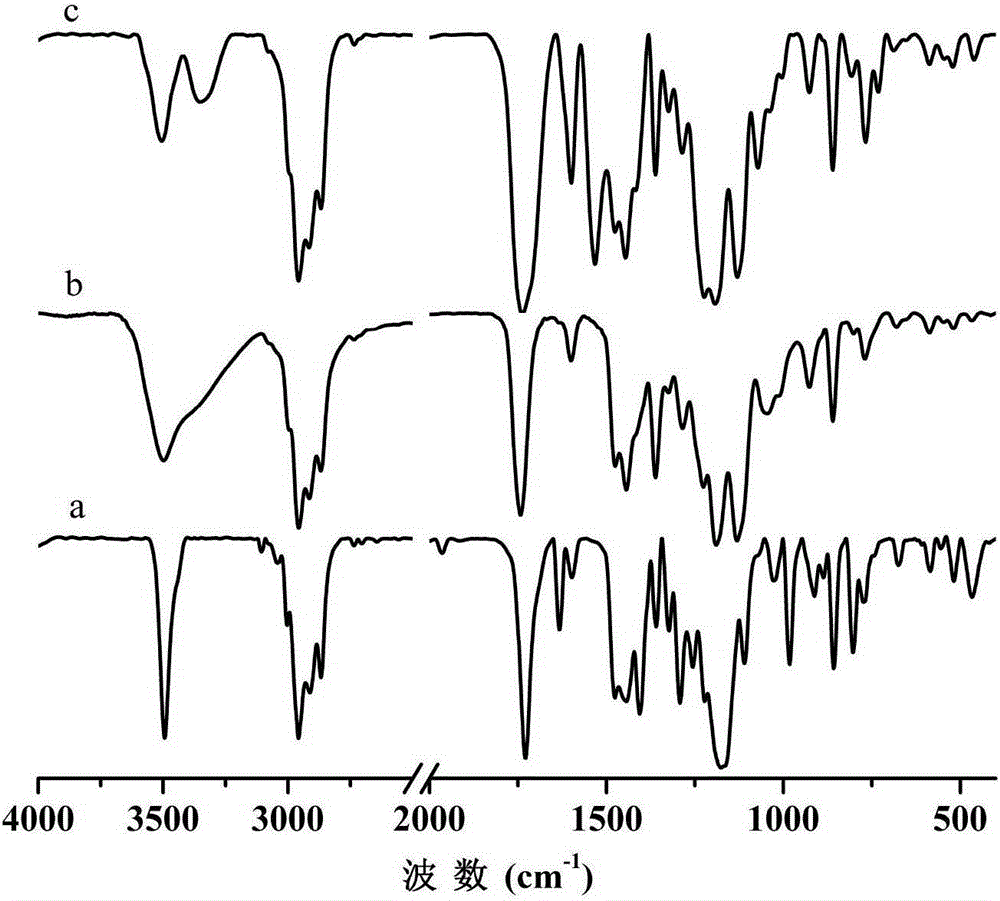
[0034] FT-IR on GM and...
Embodiment 2
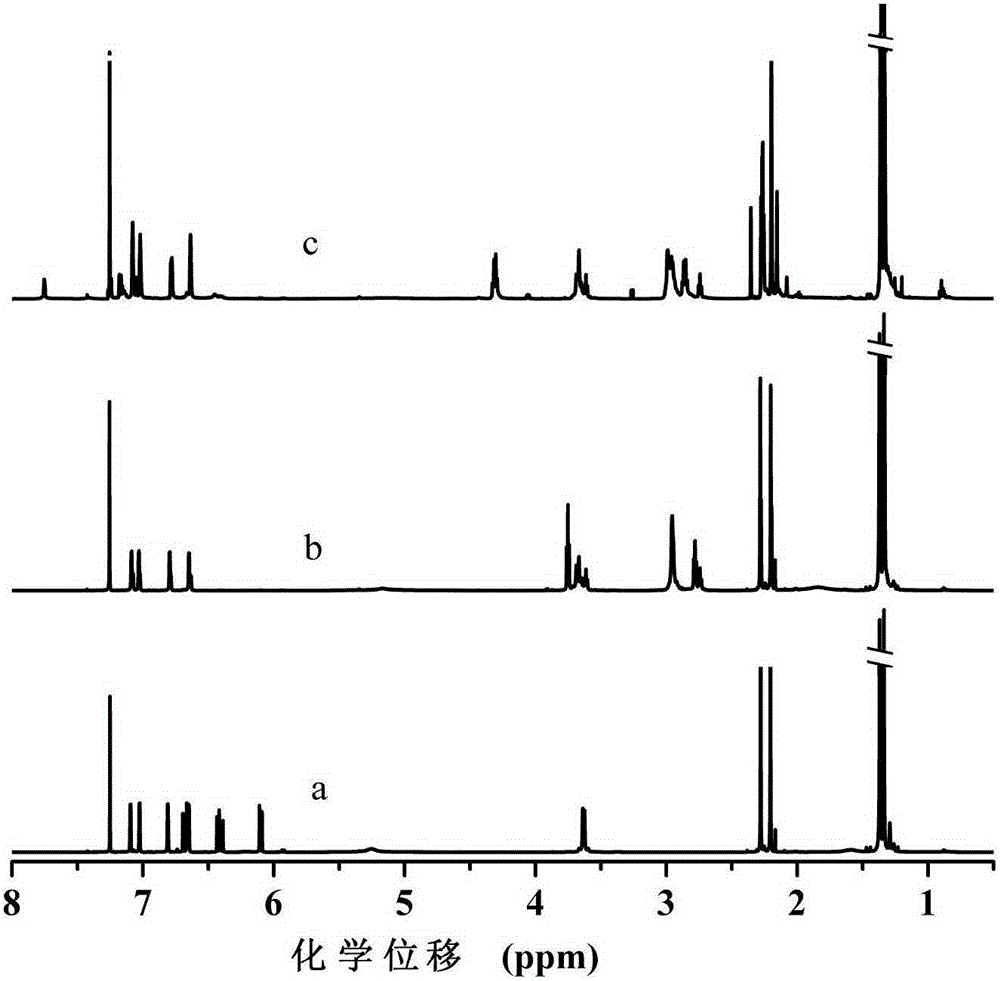
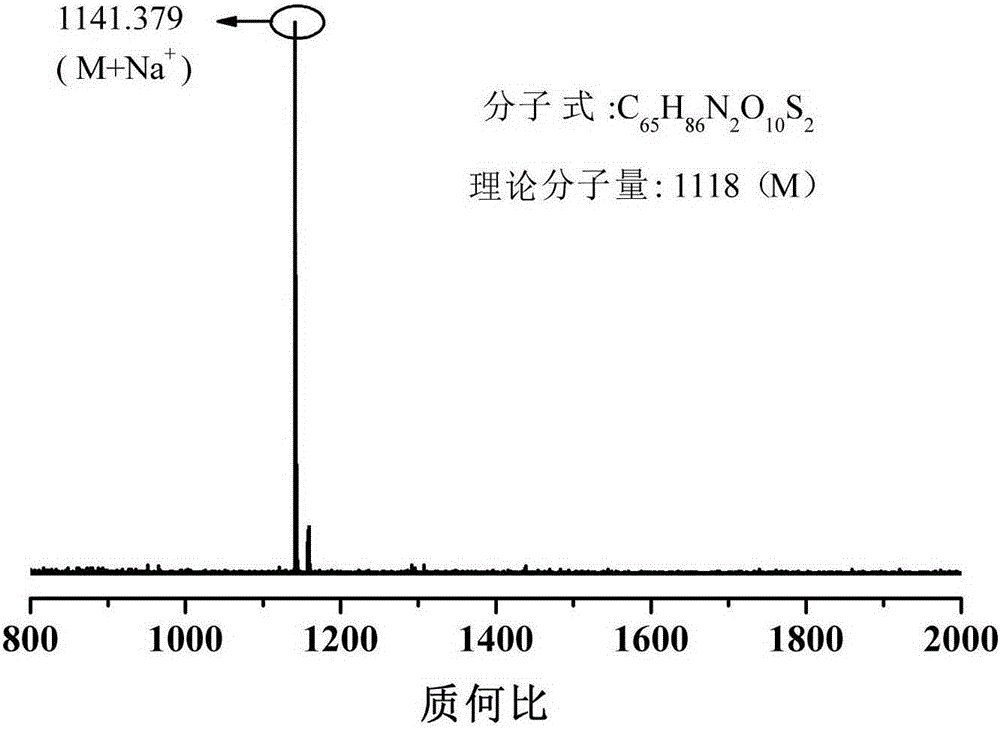
[0040] This example differs from Example 1 in that 2-mercaptoethanol is replaced by 6-mercaptohexanol (MH) in step (1), the reaction temperature is 30°C, and the reaction time is 6h; the macromolecular antioxidant yield is 94.6%. From FT-IR, 1 H-NMR and MALDI-TOF-MS analysis showed that the macromolecular hindered phenol antioxidant GM-MH-TDI containing thioether and carbamate groups was successfully prepared, the molecular weight was 1230g / mol, and its structural formula was as follows. The retention rate of mechanical properties of natural rubber vulcanizates after aging at 100°C for 72 hours is shown in Table 1. It can be seen from Table 1 that after aging at 100°C for 72 hours, 2 phr of the macromolecular hindered phenol antioxidant GM-MH-TDI synthesized in this example containing thioether and carbamate groups was added to the natural rubber vulcanizate Tensile strength and elongation retention at break were 91.2% and 80.9%, respectively. Compared with Example 1, the c...
Embodiment 3
[0043] The difference between this embodiment and Example 1 is that the toluene diisocyanate (TDI) in the step (2) is replaced by p-phenylene diisocyanate (PPDI), the consumption of dibutyltin dilaurate is 0.05mmol, and the reaction temperature is 60°C. The reaction time is 4h, and the yield of macromolecular antioxidant is 96.3%. From FT-IR, 1 H-NMR and MALDI-TOF-MS analysis showed that the macromolecular hindered phenol antioxidant GM-ME-PPDI containing thioether and carbamate groups was successfully prepared, the molecular weight was 1104g / mol, and its structural formula was:
[0044]
[0045] The retention rate of mechanical properties of the vulcanizate after aging at 100°C for 72 hours is shown in Table 1. It can be seen from Table 1 that after aging at 100°C for 72 hours, 2 phr of the macromolecular hindered phenol antioxidant GM-ME-PPDI synthesized in this example containing thioether and carbamate groups was added to the natural rubber vulcanizate Tensile strengt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com