A gene vaccine carrier, its preparation method and application
A gene vaccine and carrier technology, applied in the field of its preparation, gene vaccine carrier, to achieve the effect of mild preparation conditions, simple process and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of Small Molecular Peptides
[0037] The synthetic method step of small molecule polypeptide used in the present invention is as follows:
[0038]
[0039] Among them, Fmoc-OSu is fluorenylmethoxycarbonyl succinimide, DIPEA is N,N-diisopropylethylamine, Acetone is acetone, I 2 is iodine, triethyl phosphite is triethyl phosphite, DCM is dichloromethane, pyridine is pyridine, TFA is trifluoroacetic acid, spps is the English abbreviation of "polypeptide solid-phase synthesis", TMSBr is trimethylbromosilane, and MeOH is Methanol, methylamine hydrochloride is methylamine hydrochloride.
[0040] Step 1, Fmoc-L-Tyr-O t Synthesis of Bu
[0041] Dissolve 10mmol L-tert-butyl tyrosine and 10 molecules of N,N-diisopropylethylamine in 75mL of acetone, add 9.8mmol of Fmoc-OSu containing 75mL of acetone solution under stirring , stirred at room temperature for 12 hours, and then separated by silica gel column to obtain 4.4 g of product Fmoc-L-Tyr-O t Bu (i...
Embodiment 2
[0059] Example 2: Preparation of supramolecular hydrogel (gene vaccine carrier)
[0060] Configuration solution 1: alkaline phosphatase 2U / μL, the buffer is 50mM Tris-HCl+1mM MgCl 2 ; Solution 2: 2mgNap-GFFY(p)-NHMe (prepared in Example 1) was dissolved in 1mL PBS buffer, configured as a 2‰ (w / v) small molecule peptide solution, and Nap 2 CO 3 Adjust the pH to 9. Then, 3 μL of solution 1 was added to 100 μL of solution 2, mixed evenly, and allowed to stand at room temperature for at least 10 min to form a supramolecular hydrogel.
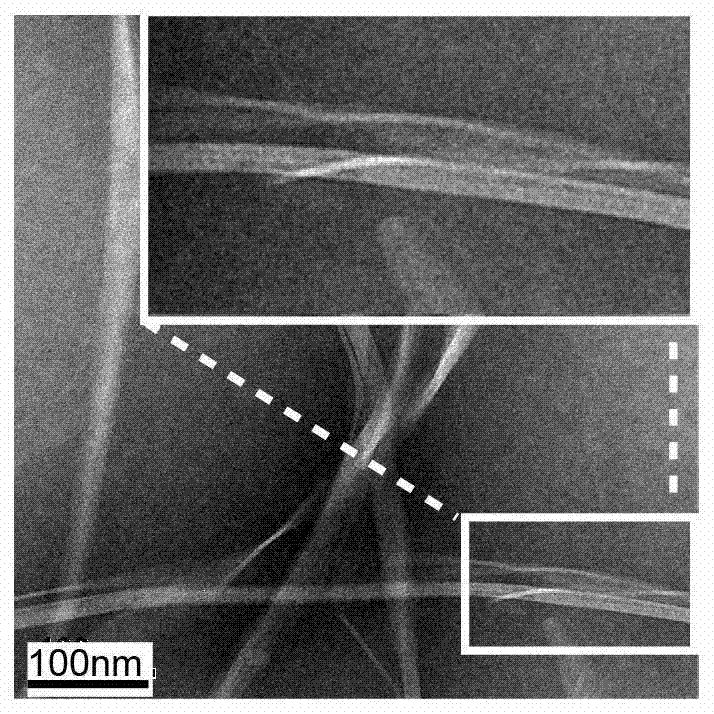
[0061] The prepared supramolecular hydrogel was observed by transmission electron microscope, such as figure 1 As shown, the prepared supramolecular hydrogel is a left-handed helix, which can compress DNA, protect its loaded DNA from degradation, and promote cell antigen expression.
Embodiment 3
[0062] Example 3: Preparation of supramolecular hydrogel loaded with HIV DNA (gene vaccine carrier loaded with DNA)
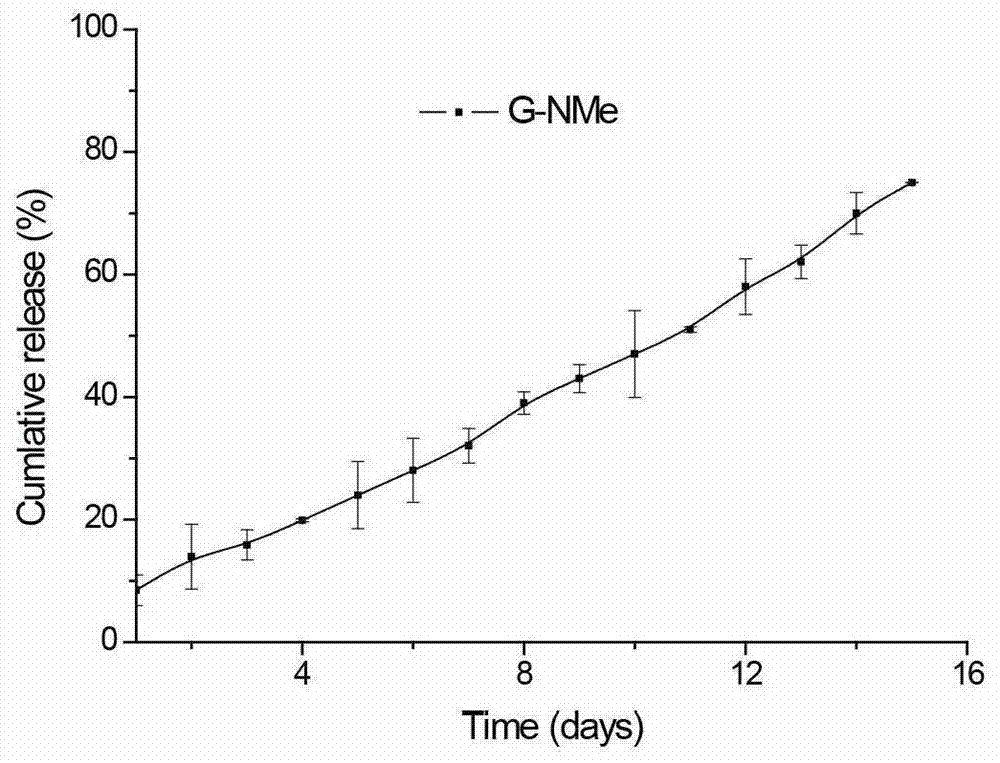
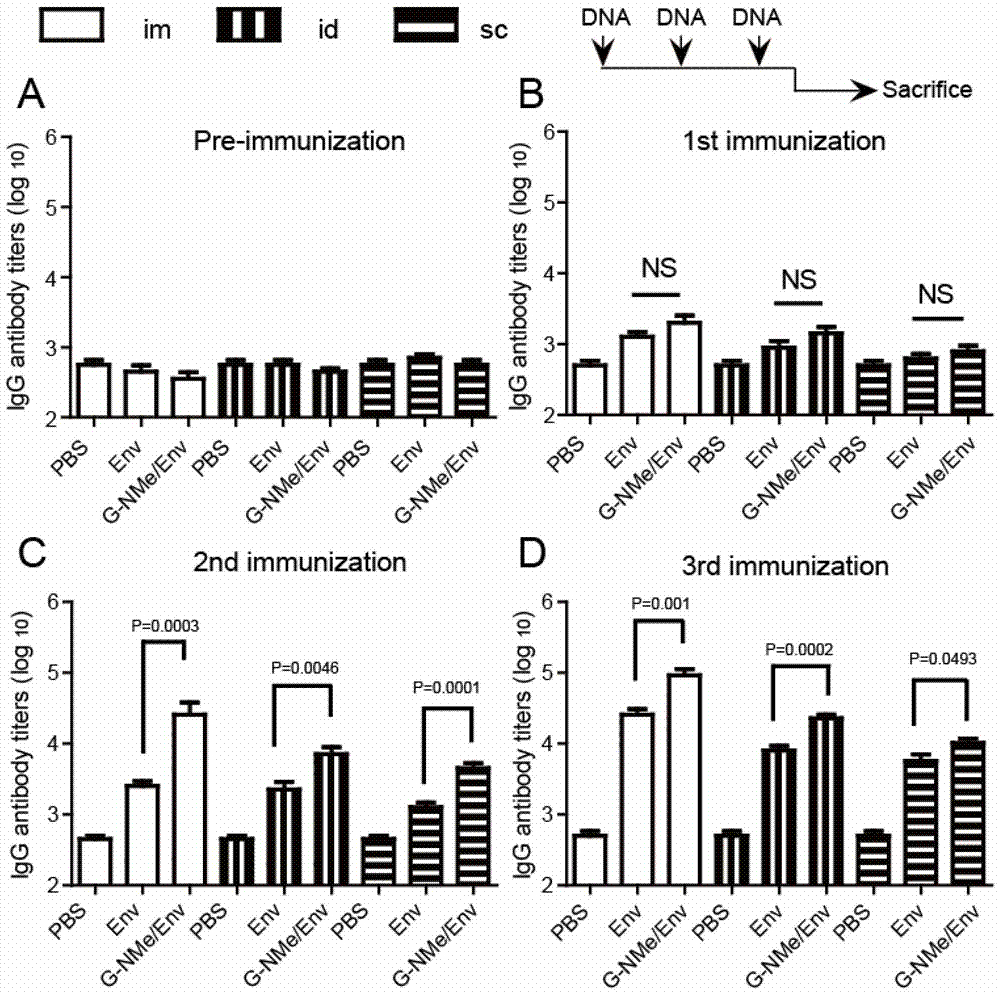
[0063] Solution 1: Alkaline phosphatase 2U / μL, the buffer is 50mM Tris-HCl, 1mM MgCl2; Solution 2: Dissolve 2mgNap-GFFY(p)-NHMe (prepared in Example 1) in 1mL PBS buffer, and configure into a 2‰ (w / v) small molecule peptide solution, with Na 2 CO 3 Adjust the pH to 9, and add 10 μg of HIV DNA vaccine (pDRVI SV1.0-Env). Then, 3 μL of solution 1 was added to 100 μL of solution 2, mixed evenly, and allowed to stand at room temperature for at least 10 min to form a supramolecular hydrogel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com