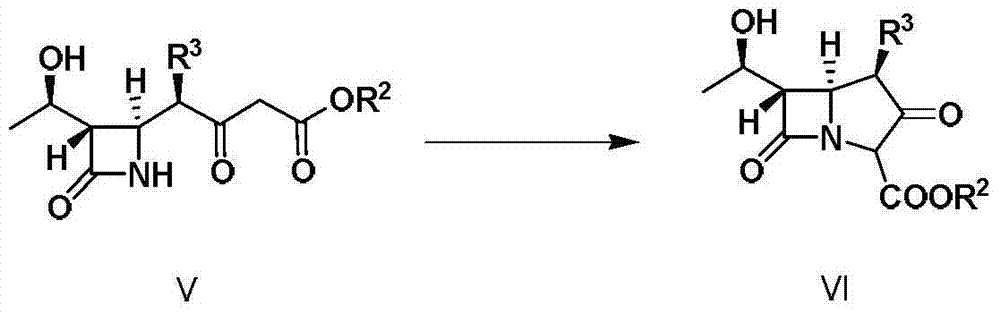
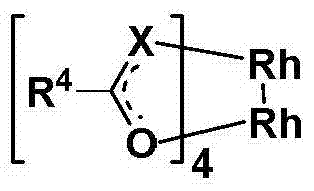
Carboxyl-containing polymer, preparation method and application thereof, as well as preparation method of supported catalyst and imipenem antibiotic intermediate
A polymer and supported technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve heavy metal loss, production Problems such as high cost and poor mechanical properties can achieve the effect of reducing loss, improving mechanical properties and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Synthetic monomer
[0090]
[0091] Add 4-vinylbenzyl alcohol (1,20 g, 149 mmol), dodecanedioic acid (2,30 g, 149 mmol) and 4-dimethylaminopyridine (1.82 g, 14.9 mmol) to tetrahydrofuran (300 mL) at room temperature Dicyclohexylcarbodiimide (32.1 g, 156 mmol) was added in portions to the solution. Stirring was continued at room temperature for 6 hours, and the insoluble matter was removed by filtration. The filtrate was concentrated, and the residue was dissolved in ethyl acetate (300 mL), washed with 1N hydrochloric acid (100 mL×2), dried over anhydrous sodium sulfate, and then concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain compound 3 (22.4 g, 43% yield) and compound 4 (15.6 g, 45% yield).
[0092] Compound 3: 1 H NMR (CDCl 3 ,400MHz): δ1.24–1.36(m,12H),1.57–1.68(m,4H),2.31–2.38(m,4H),5.10(s,2H),5.26(d,J=10.9Hz,1H ), 5.75(d, J=17.6Hz, 1H), 6.71(dd, J=17.6, 10.9Hz, 1H), 7.31(d, J=8.1Hz, 2H), 7.40(d, J...
Embodiment 2
[0100] Synthetic monomer
[0101]
[0102] Add 4-vinylbenzyl chloride (7, 5.0 g, 31.92 mmol) in ethanol slowly to monomethylamine in ethanol (33.3%, 150 mL, 1.6 mol) at 40-45°C. After the reaction was complete, the reaction mixture was cooled, and potassium carbonate (6.62 g, 47.9 mmol) was added to continue stirring for 1 hour. Solid was removed by filtration, and the filtrate was concentrated to obtain the crude product of red oil compound 8 ( 1 H NMR yield: 52%), directly used in the next reaction.
[0103] Compound 8: 1 H NMR (CDCl 3 ,400MHz): δ2.45(s,3H),3.74(s,2H),5.22(d,J=10.9Hz,1H),5.73(d,J=17.6Hz,1H),6.71(dd,J= 17.6, 10.9Hz, 1H), 7.27(d, J=7.9Hz, 2H), 7.37(d, J=7.9Hz, 2H).
[0104] At room temperature, dodecanedioic acid (2, 20.6g, 89.5mmol) was dissolved in tetrahydrofuran (350mL), dicarbonyl imidazole (29.0g, 179mmol) was added in batches, stirring was continued for 1 hour, and then compound 8 (12.5 g, 1 H NMR content, 84.9 mmol). React at room temperatu...
Embodiment 3
[0113] Synthetic monomer
[0114]
[0115] Synthesis of Compound 13
[0116] At room temperature, 8-bromo-1-octanol (12, 11.0g, 52.6mmol), dihydropyran (22.1g, 263mmol) and pyridinium p-toluenesulfonate (528.7mg, 2.1mmol) were successively added to the reaction flask ) in dichloromethane (110 mL), stirred at room temperature until the reaction was complete. The reaction solution was concentrated until there was no fraction, and the crude product was purified by silica gel column chromatography to obtain compound 13 (14.1 g, 89% yield) as a yellow oil.
[0117] Compound 13: 1 H NMR (CDCl 3 ,400MHz): δ1.28–1.46(m,8H),1.48–1.62(m,6H),1.66–1.74(m,1H),1.79–1.87(m,3H),3.34–3.41(m,3H) ,3.46–3.51(m,1H),3.69–3.75(m,1H),3.83–3.88(m,1H),4.55–4.57(m,1H).
[0118] Synthesis of compound 16
[0119] Under ice-water bath, the DMF (2mL) solution of 4-vinylbenzyl alcohol (1,4.0g, 29.8mmol) was added in the DMF (36mL) suspension of NaH (60% content, 1.30g, 32.4mmol), and then in Stir fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com