Triiodothyroxin hapten luminous marker and synthetic method thereof
A technology of luminescent markers and thyroxine, which is applied in the direction of organic chemistry, can solve the problems of high price and easy to produce differences, and achieve the effect of easy purification and improved detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be described in further detail below in conjunction with specific embodiments.
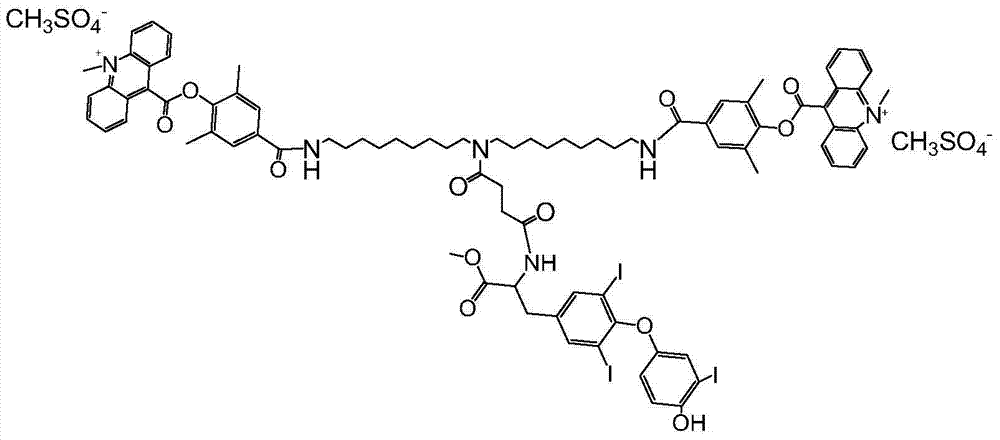
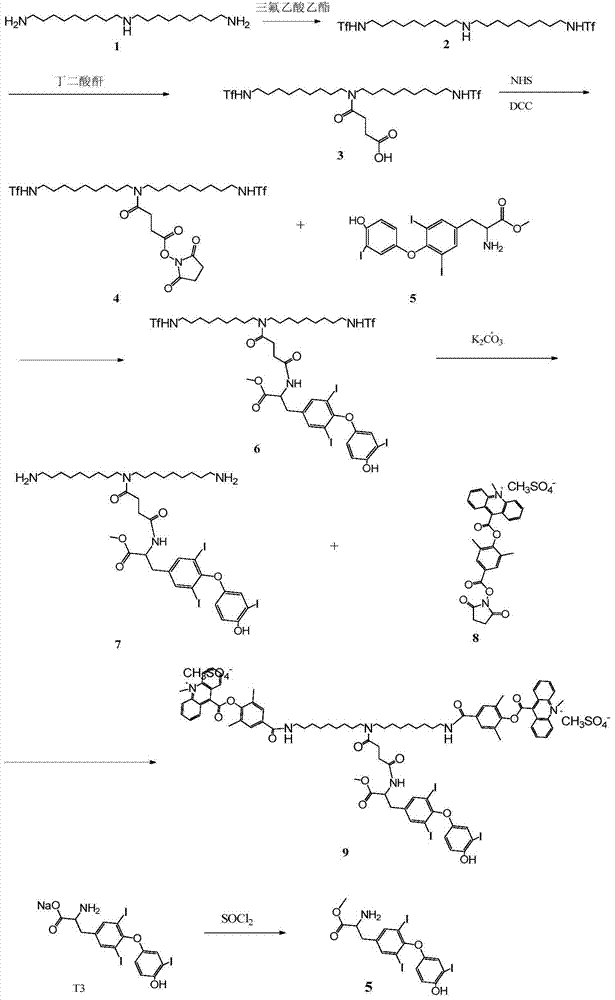
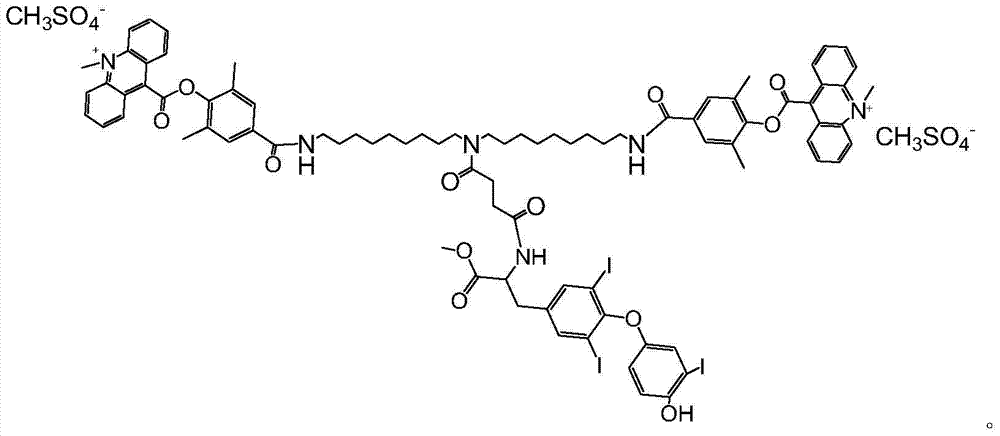
[0020] The synthetic route of the novel compound used for the detection of triiodothyronine by chemiluminescence method according to the present invention is as follows:
[0021]
[0022] specific:
[0023] Preparation of compound 2
[0024] 11.55 g of bisnonyltriamine was dissolved in 100 ml of acetonitrile, and 1.56 g of ethyl trifluoroacetate was added.
[0025] 1. Stir overnight at room temperature.
[0026] 2. Monitor the completion of the reaction by thin layer chromatography.
[0027] 3. Concentrate under reduced pressure and pump to dryness to obtain 2 g of crude product of trifluoroacetamide compound 2 (Formula: C20H39F6N3O4S2, MW: 563.66).
[0028] Preparation of compound 3
[0029] 1. Dissolve the crude product of trifluoroacetamide (compound 2) in 100ml of acetonitrile, and first add 0.875ml of triethylamine.
[0030] 2.0.74g of succinic anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com