Preparation method of potassium ferrate
A technology of potassium ferrate and potassium hydroxide, which is applied in the fields of chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problem of high air tightness and corrosion resistance requirements of production equipment, complex purification process of potassium ferrate, and impractical Better and complete separation of problems, to achieve the effect of easy filtration and removal, elimination of potential safety hazards, and improvement of safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
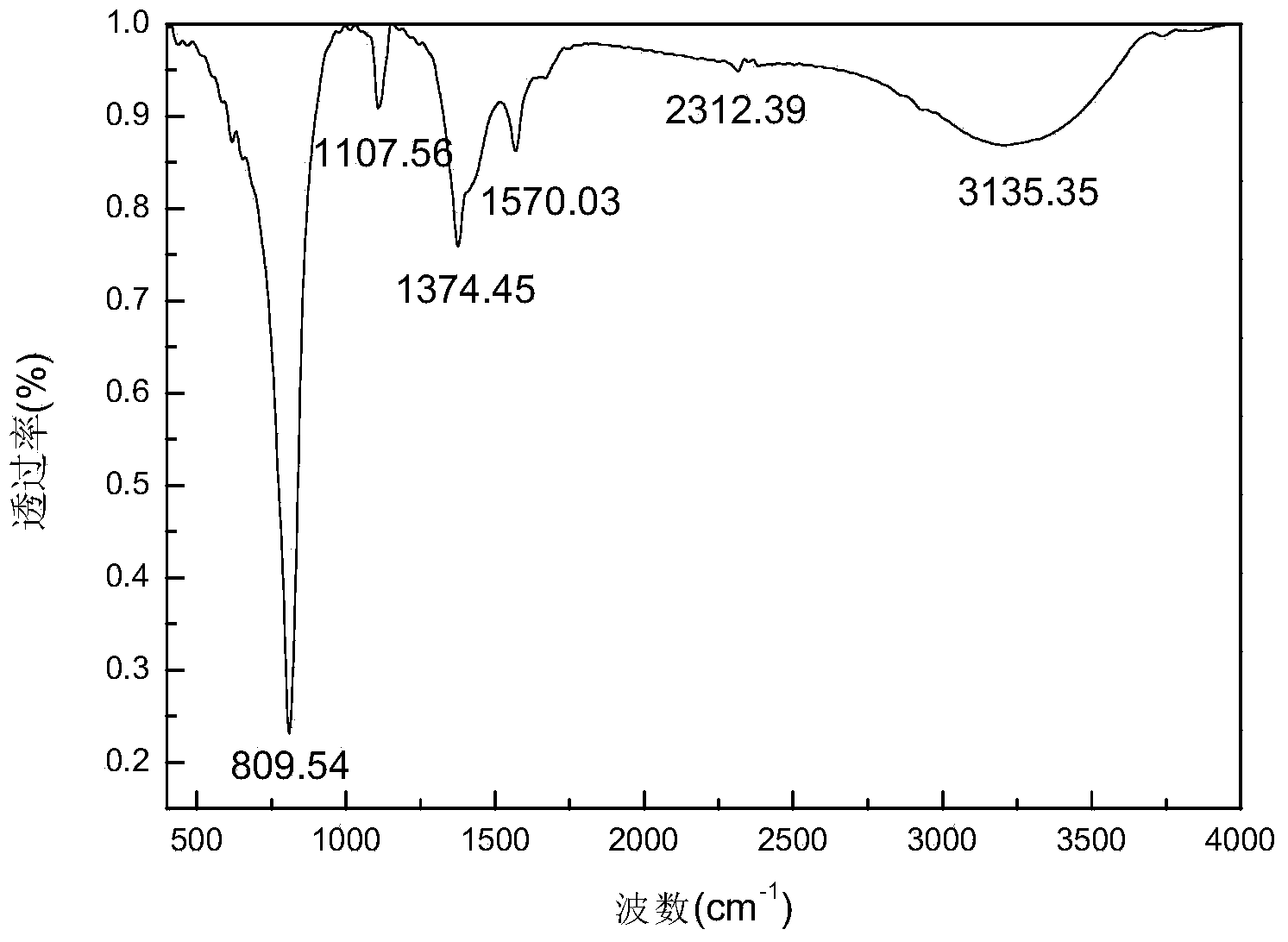
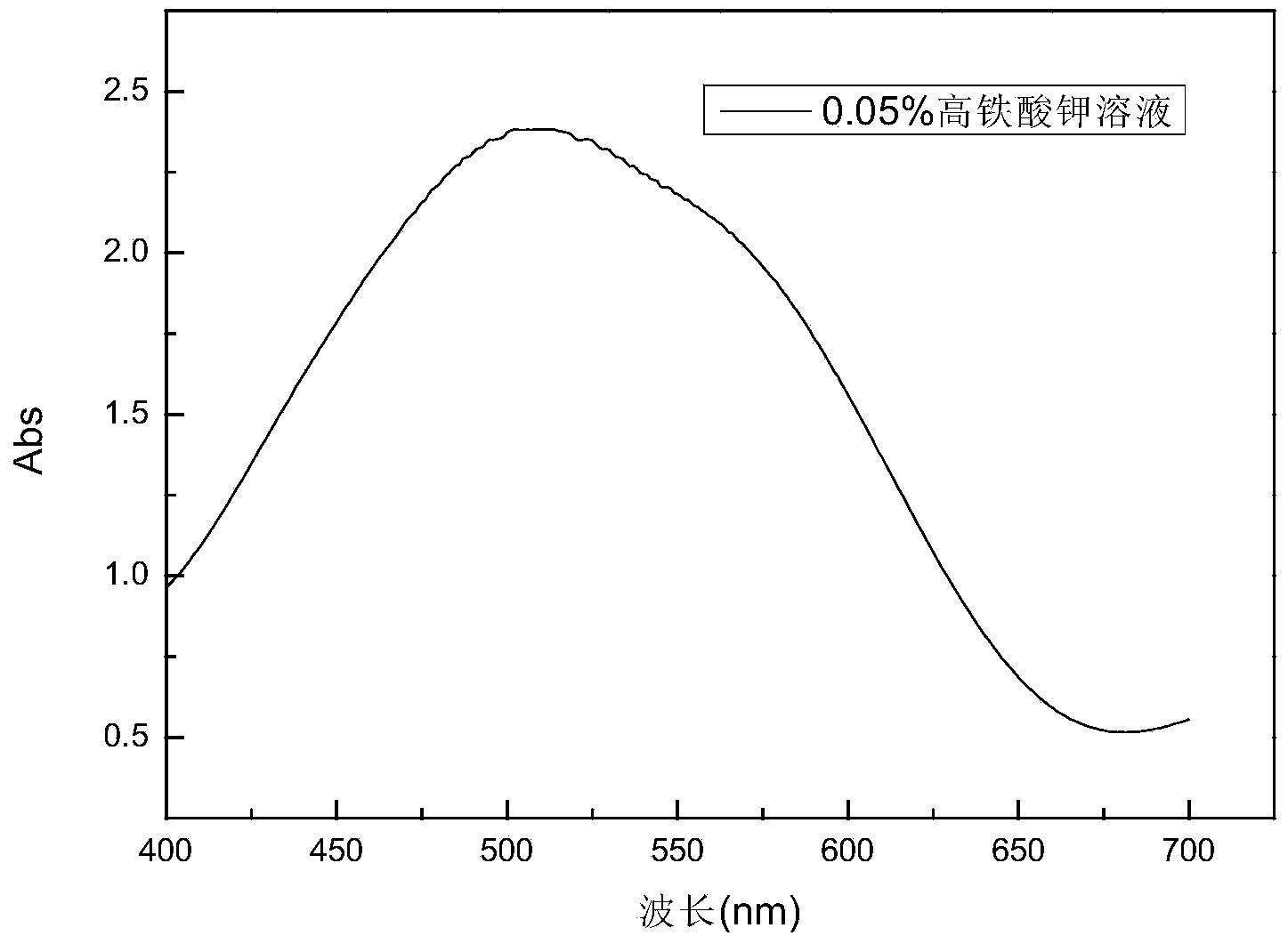
Image
Examples
Embodiment 1
[0026] (1) Weigh 8.00g of calcium hypochlorite and 5.33g of potassium carbonate respectively, and configure them into equal-volume solutions of 50ml each, mix the two, and after reacting for 15min, filter to remove the calcium carbonate precipitate, place the filtrate in an ice-water bath, and Stir while slowly adding potassium hydroxide solid 100g to the filtrate to obtain an alkaline saturated potassium hypochlorite solution;
[0027] (2) In an ice-water bath environment, slowly add 20.79 g of ferric nitrate nonahydrate solid into the alkaline saturated potassium hypochlorite solution prepared in step (1), stir at a speed of 900 r / min, and react for 60 minutes to generate potassium ferrate solution ;
[0028] (3) Add 100ml of saturated potassium hydroxide solution to the potassium ferrate solution prepared in step (2), leave standstill in the ice-water bath environment for 30min, and filter with G2 glass sand core funnel to obtain the solid thick product of potassium ferrate...
Embodiment 2
[0032] (1) Weigh 12.00g of calcium hypochlorite and 8.00g of potassium carbonate respectively, and configure each 100ml of equal-volume solutions, mix the two, and after reacting for 15min, filter to remove the calcium carbonate precipitate, place the filtrate in an ice-water bath, and Stir, slowly add potassium hydroxide solid 200g in the filtrate, obtain alkaline saturated potassium hypochlorite solution;
[0033] (2) In an ice-water bath environment, slowly add 31.20 g of ferric nitrate nonahydrate solid into the alkaline saturated potassium hypochlorite solution prepared in step (1), stir at a speed of 900 r / min, and react for 60 minutes to generate potassium ferrate solution ;
[0034] (3) Add 200ml of saturated potassium hydroxide solution to the potassium ferrate solution prepared in step (2), after leaving standstill in the ice-water bath environment for 30min, filter with G2 glass sand core funnel to obtain the solid thick product of potassium ferrate;
[0035] (4) D...
Embodiment 3
[0038] (1) Weigh 18.00g of calcium hypochlorite and 12.00g of potassium carbonate respectively, and configure each 100ml of equal-volume solutions, mix the two, react for 15min, filter and remove the calcium carbonate precipitate, place the filtrate in an ice-water bath, and Stir, slowly add potassium hydroxide solid 200g in the filtrate, obtain alkaline saturated potassium hypochlorite solution;
[0039] (2) In an ice-water bath environment, slowly add 46.80 g of ferric nitrate nonahydrate solid to the alkaline saturated potassium hypochlorite solution prepared in step (1), stir at a speed of 900 r / min, and react for 60 minutes to generate potassium ferrate solution ;
[0040] (3) Add 200ml of saturated potassium hydroxide solution to the potassium ferrate solution prepared in step (2), after leaving standstill in the ice-water bath environment for 30min, filter with G2 glass sand core funnel to obtain the solid thick product of potassium ferrate;
[0041] (4) Dissolve the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com