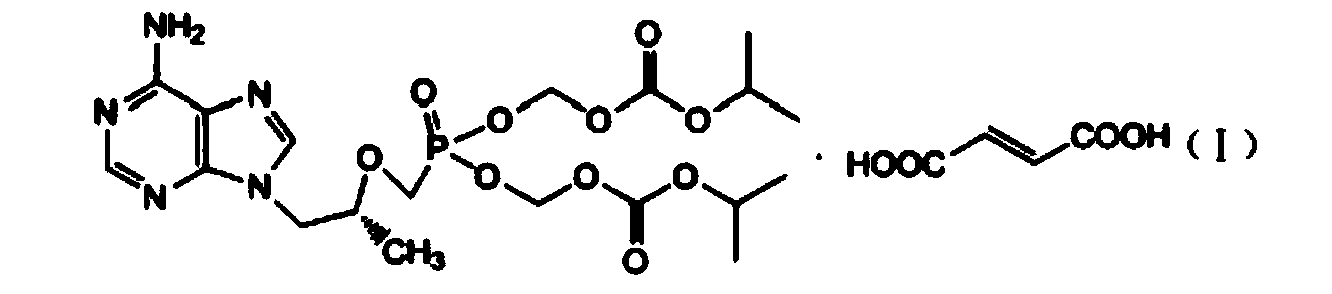
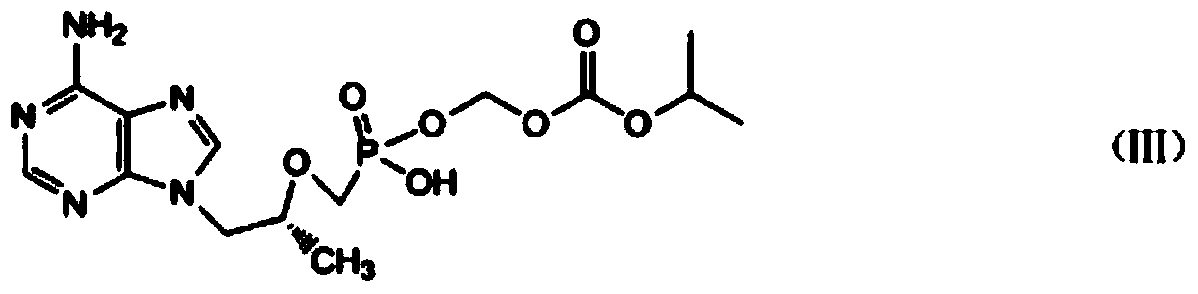
Preparation method of high-purity fumaric acid tenofovir disoproxil fumarate
A technology of tenofovir disoproxil fumarate and tenofovir disoproxil fumarate, which is applied in the field of preparation of high-purity tenofovir disoproxil fumarate, can solve the problem of large number of product impurities, loss of tenofovir disoproxil, Eliminate impurities and troubles, and achieve the effects of reducing the production of three wastes, reducing the reaction process, and increasing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of the tenofovir disoproxil fumarate of the present embodiment may further comprise the steps:
[0033] (1) preparing tenofovir, specifically comprising the following steps:
[0034] 1a. prepare R-9-(2-hydroxypropyl) adenine, add adenine, R-propylene carbonate, dimethylformamide and sodium hydroxide in reaction vessel, adenine, R-propylene carbonate and The molar ratio of sodium hydroxide is 140:188:8, the ratio of sodium hydroxide and dimethylformamide is 8 moles: 80L, stir evenly, after reaction is complete, purify, dry, obtain R-9-(2-hydroxyl Propyl) adenine;
[0035] 1b. prepare R-9-[2-(diethylphosphorylmethoxy) propyl] adenine, add R-9-(2-hydroxypropyl) adenine, magnesium tert-butoxide and The ratio of isopropanol, magnesium tert-butoxide, isopropanol to adenine is 160 moles: 200L: 140 moles, the temperature is raised to 50°C, and the ratio of 125 moles: 100L p-toluenesulfonyloxy N-methylpyrrolidone solution of diethyl methyl phosphate, th...
Embodiment 2
[0043] The preparation method of the tenofovir disoproxil fumarate of the present embodiment may further comprise the steps:
[0044] (1) preparing tenofovir, specifically comprising the following steps:
[0045] 1a. Prepare R-9-(2-hydroxypropyl) adenine, add 148mol adenine, 192mol R-propylene carbonate, 90L dimethylformamide and 11.75mol sodium hydroxide in the reaction vessel, stir evenly, and the reaction is complete Afterwards, purify and dry to obtain R-9-(2-hydroxypropyl)adenine;
[0046] 1b. Prepare R-9-[2-(diethylphosphorylmethoxy)propyl]adenine, add the hydroxypropyl)adenine prepared in step 1a, 165.4mol magnesium tert-butoxide and 220L Isopropanol, heat up to 70°C, add dropwise the N-methylpyrrolidone solution of diethyl p-toluenesulfonyloxymethyl phosphate into the reaction vessel, the amount of diethyl p-toluenesulfonyloxymethyl phosphate added is 130.3 mol, the water content of N-methylpyrrolidone is less than 0.1%, and its addition amount is 120L; the isopropan...
Embodiment 3
[0053] The preparation method of the tenofovir disoproxil fumarate of the present embodiment may further comprise the steps:
[0054] (1) preparing tenofovir, specifically comprising the following steps:
[0055] 1a. prepare R-9-(2-hydroxypropyl) adenine, add adenine, R-propylene carbonate, dimethylformamide and sodium hydroxide in reaction vessel, adenine, R-propylene carbonate and The molar ratio of sodium hydroxide is 155:196:13, the ratio of sodium hydroxide and dimethylformamide is 13 moles: 100L, stir evenly, after reaction is complete, purify, dry, obtain R-9-(2-hydroxyl Propyl) adenine;
[0056] 1b. prepare R-9-[2-(diethylphosphorylmethoxy) propyl] adenine, add R-9-(2-hydroxypropyl) adenine, magnesium tert-butoxide and The ratio of isopropanol, magnesium tert-butoxide, isopropanol to adenine is 170 moles: 250L: 155 moles, the temperature is raised to 90°C, and the ratio of 133 moles: 150L p-toluenesulfonyloxy N-methylpyrrolidone solution of diethyl methyl phosphate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com