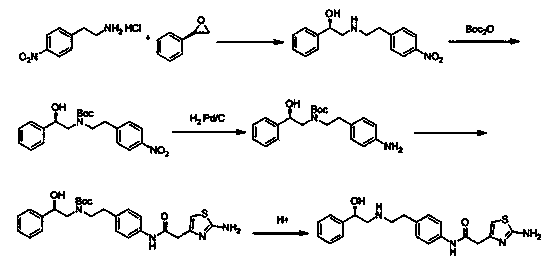
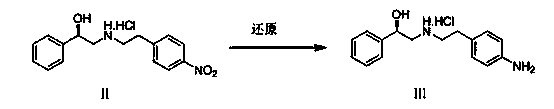
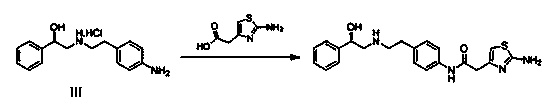Synthesis method of mirabegron
A synthesis method and compound technology, applied in the field of synthesis, can solve problems such as not suitable for large-scale industrial production, high impurity content, complicated operation, etc., and achieve the effect of avoiding the use of high boiling point solvents, reducing impurity content, and reducing solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, a kind of synthetic method of mirabegron
[0029] Including the following steps:
[0030] step 1)
[0031] Into a 50L glass reactor, add 1.01kg of R-mandelic acid, 1.4kg of 4-nitrophenethylamine hydrochloride, 0.1kg of HOBt, 6L of DMF; slowly add 0.7kg of triethylamine dropwise while controlling the internal temperature to ≤25°C; After the dropwise addition is completed, add EDC in batches. After the addition is complete, continue to stir for 1-2 hours; TLC monitors the completion of the reaction, add 14L ethyl acetate and 28L water, stir for 30 minutes, let stand to separate, and use 1mol / L hydrochloric acid solution for the organic phase 15L was washed once, and 20% (w / w) potassium carbonate aqueous solution was washed twice (15L each time); the organic phase was dried, suction filtered, and concentrated to obtain the crude product; the crude product was recrystallized with toluene to obtain a white solid (intermediate compound I) 1.90kg , yield 9...
Embodiment 2
[0038] Embodiment 2, a kind of synthetic method of mirabegron
[0039] Including the following steps:
[0040] step 1)
[0041] Into a 50L glass reactor, add 1.01kg of R-mandelic acid, 1.4kg of 4-nitrophenethylamine hydrochloride, 0.1kg of HOBt, 6L of DMF; slowly add 0.7kg of triethylamine dropwise while controlling the internal temperature to ≤25°C; After the dropwise addition is completed, add EDC in batches. After the addition, continue to stir for 1-2 hours; TLC monitors the completion of the reaction; add 14L ethyl acetate and 28L water, stir for 30 minutes, let stand to separate, and use 1mol / L hydrochloric acid solution for the organic phase 15L was washed once, and 20% (w / w) potassium carbonate aqueous solution was washed twice (each time 15L); the organic phase was dried, suction filtered, and concentrated to obtain the crude product; the crude product was recrystallized with toluene to obtain a white solid (intermediate compound I) 1.95kg , yield 93%, content...
Embodiment 3
[0048] Embodiment 3, a kind of synthetic method of mirabegron
[0049] Including the following steps:
[0050] step 1)
[0051] Add 1.01kg of R-mandelic acid, 1.4kg of 4-nitrophenethylamine hydrochloride, 0.2kg of HOBt, and 6L of DMF into a 50L glass reactor; slowly add 0.7kg of triethylamine dropwise while controlling the internal temperature to ≤25°C; After the dropwise addition is completed, add EDC in batches. After the addition, continue to stir for 1-2 hours; TLC monitors the completion of the reaction; add 14L ethyl acetate and 28L water, stir for 30 minutes, let stand to separate, and use 1mol / L hydrochloric acid solution for the organic phase 15L was washed once, and 20% (w / w) potassium carbonate aqueous solution was washed twice (15L each time); the organic phase was dried, suction filtered, and concentrated to obtain the crude product; the crude product was recrystallized with toluene to obtain a white solid (intermediate compound I) 1.92kg , yield 91%, cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com