Protein-free culture medium for culturing microencapsulated recombinant Chinese hamster ovary (CHO) cells and preparation method thereof
A protein-free culture medium and ovarian cell technology, which is applied in the field of protein-free culture medium for microencapsulated recombinant Chinese hamster ovary cell culture, can solve problems such as high price, and achieve the effects of simple operation, low price and avoiding potential pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Prepare protein-free medium for microencapsulated recombinant CHO cells:
[0035] Culture medium of the present invention is made up of two parts: DMEM / F12 culture medium (purchased from Sigma Company, composition sees Table 1) and following composition:
[0036]
[0037] Table 1 DMEM / F12 medium composition
[0038]
[0039] All the above substances are chemical reagents of analytical grade, dissolved in ultrapure water with a final volume of 90%, and after fully stirring for half an hour, dilute to the required final volume and keep for 10 minutes.
[0040] Sterile filtration is carried out according to the standard operation of flat filter.
[0041] 2. Preparation of microencapsulated recombinant CHO cells:
[0042] Reference literature (document 4. Ma X J, 1994. Generation of alginate-poly-Lysine alginate (APA) biomicrocapsules: the relationship between the membrane strength and the reaction conditions. Art Cells Blood Subs and Immob Biotech, 22 (1): 43- ...
Embodiment 2
[0045] 1. Prepare protein-free medium for microencapsulated recombinant CHO cells:
[0046] Culture medium of the present invention is made up of two parts: DMEM / F12 culture medium (purchased from Sigma Company, composition sees Table 1) and following composition:
[0047]
[0048] 2. Preparation of microencapsulated recombinant CHO cells (same as Example 1):
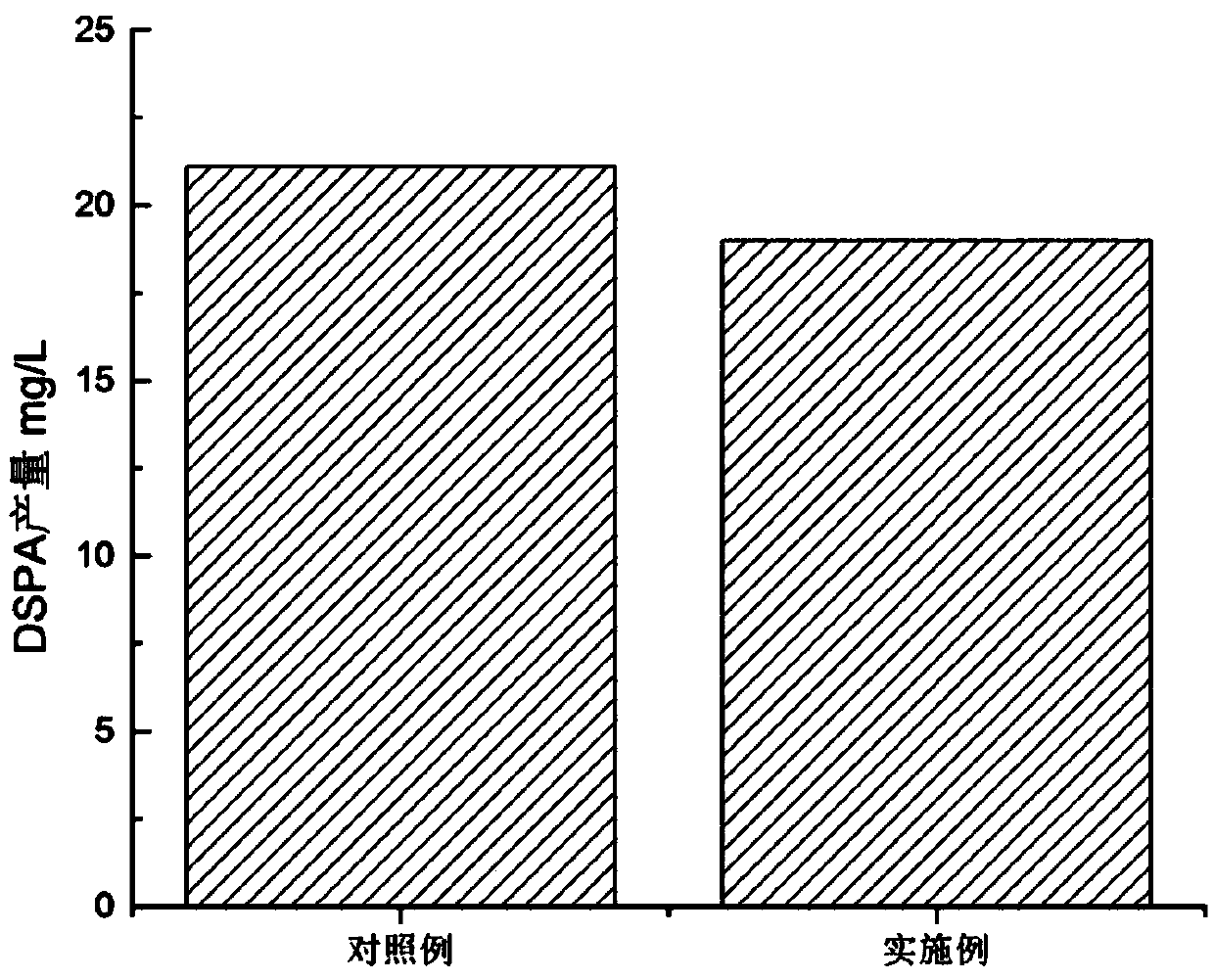
[0049] Microencapsulate cells at 37 °C, 5% CO 2 In an incubator, culture in the medium prepared in the above step 1 of this embodiment for 7 days to measure the expression level of DSPA, and compare it with the control example.
Embodiment 3
[0051] 1. Prepare protein-free medium for microencapsulated recombinant CHO cells:
[0052] Culture medium of the present invention is made up of two parts: DMEM / F12 culture medium (purchased from Sigma Company, composition sees Table 1) and following composition:
[0053]
[0054] 2, the preparation of empty microcapsules (do not add cell, all the other are with embodiment 1):
[0055] microcapsules at 37 °C, 5% CO 2 In an incubator, culture the microcapsules for 7 days in the medium prepared in step 1 of this embodiment to observe the stability of the microcapsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com