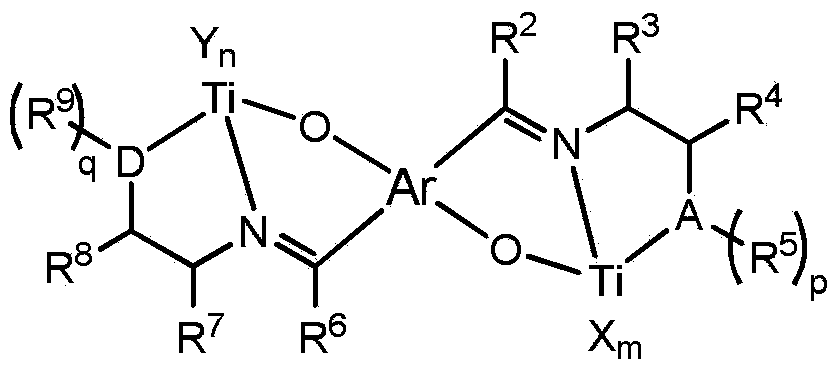
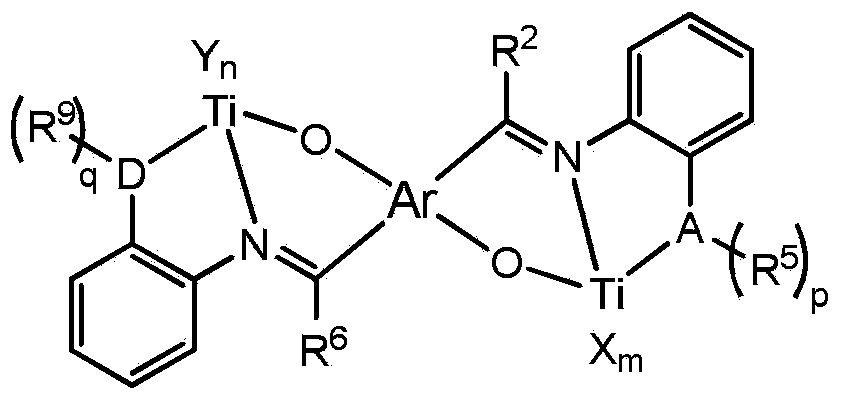
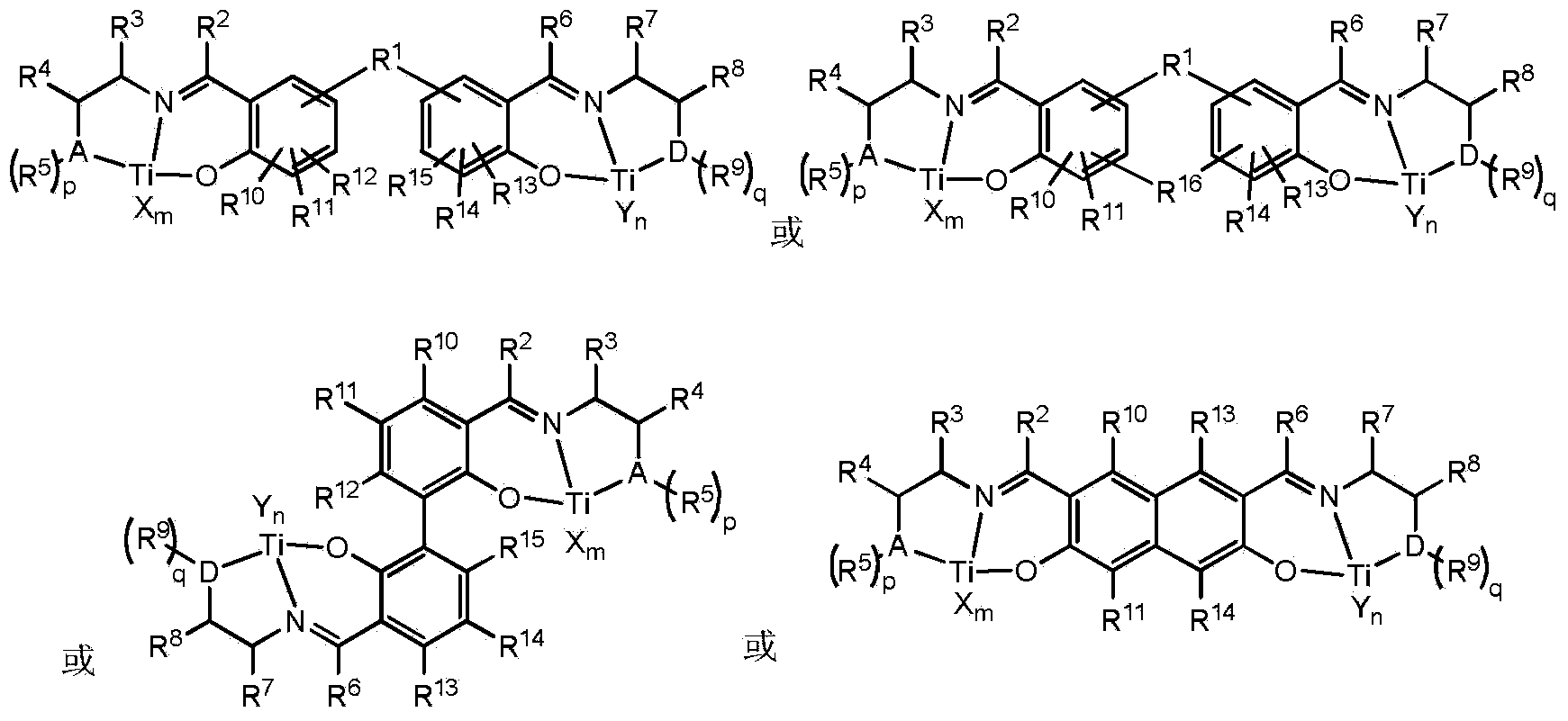
Non-metallocene tridentate binuclear titanium complex, preparation method and purpose thereof
A titanium complex, non-locene technology, applied in the field of non-locene tridentate binuclear titanium complexes, can solve the problems of polymer molecular weight increase, molecular weight distribution widening, bimetallic structure asymmetry, etc., to achieve mild reaction conditions, High polymerization activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Ligand L1
[0040]
[0041] Add 10.01mol of biphenyl salicylaldehyde D, 0.01mol of o-phenoxyaniline and 50ml of toluene solvent in the three-necked flask, 0.02g of p-toluenesulfonic acid as a catalyst, heat up and reflux for 12 hours, then add 0.01mol of o-propylthioaniline, and then After reflux for 12 hours, the reaction was stopped, and the product was separated by silica gel column chromatography to obtain ligand L1 with a yield of 18%. 1 HNMR (400MHz, CDCl 3 ): δ8.87(2H, s, N=CH), 7.68-7.14(19H, m, Ph-H), 5.35(2H, s, O-H), 2.94(2H, t, CH 2 ),1.35(2H,m,CH 2 ),0.90(3H,t,CH 3 ); Elemental analysis: theoretical value (%): C, 75.24; H, 5.41; N, 5.01; test value (%): C, 75.05; H, 5.68; N, 4.81.
Embodiment 2
[0043] Synthesis of Ligand L2
[0044]
[0045] The synthesis method is as shown in Example 1, and the ligand L2 is obtained with a yield of 22%. 1 HNMR (400MHz, CDCl 3 ):δ7.78-7.25(23H,m,Ph-H),5.35(2H,s,O-H),3.96(2H,s,CH 2 ),3.58(1H,s,O-H),1.72-1.43(10H,m,C-H),1.81(3H,s,CH 3 ),1.35(18H,s,C(CH 3 ) 3 ); Elemental analysis: Theoretical value (%): C, 79.58; H, 7.30; N, 3.44; Test value (%): C, 79.96; H, 7.23; N, 3.51.
Embodiment 3
[0047] Synthesis of Ligand L3
[0048]
[0049] Add 0.01mol of 4,8-diisopropyl-3,7-dihydroxy-2,6-anthracenedicarbaldehyde D3, 0.02mol of 2-hexeselenopropylamine, 2 drops of glacial acetic acid, and 60ml of toluene into the three-necked flask As a solvent, the water trap was refluxed for 36 hours under the protection of nitrogen, and the reaction was monitored by TLC. The reaction was stopped and the solvent was spin-dried. The product L3 was obtained by recrystallization from a mixed solvent of ethanol and water. Yield: 56.5%. 1 HNMR (400MHz, CDCl 3 ):δ8.56(2H,s,N=CH),8.34-8.20(4H,m,Ph-H),5.38(2H,s,O-H),3.70(4H,d,N-CH 2 ),2.87(2H,m,CH),1.70-0.88(46H,m,CH n ); Elemental analysis: Theoretical value (%): C, 63.31; H, 7.97; N, 3.69; Test value (%): C, 63.45; H, 7.51; N, 3.63.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com