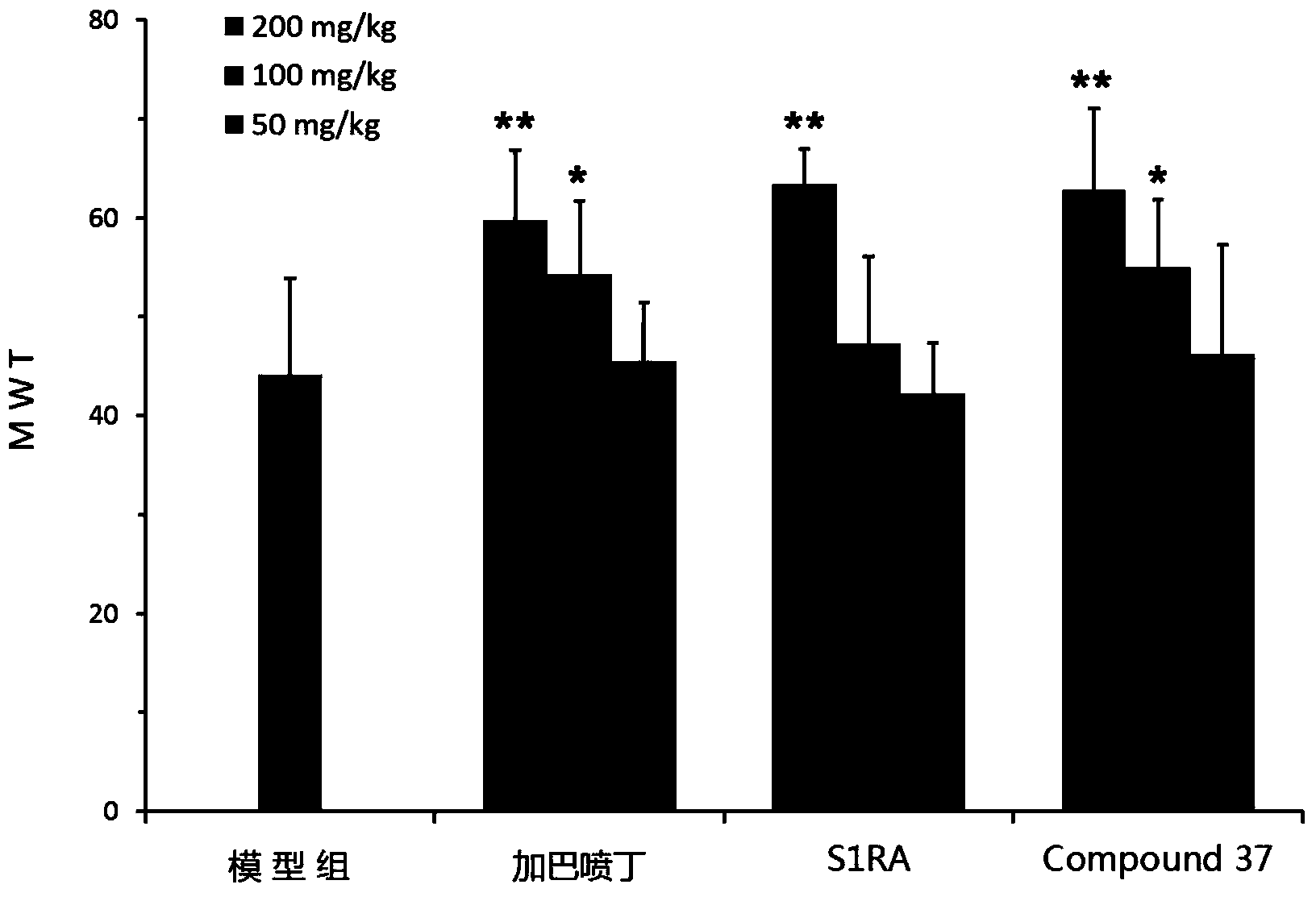
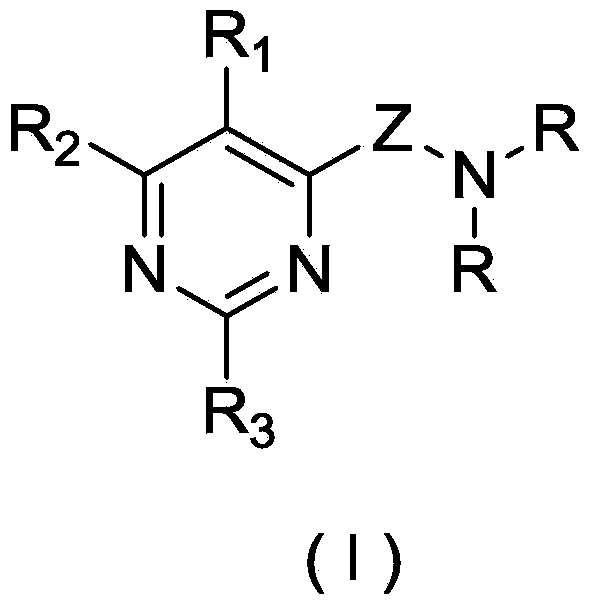
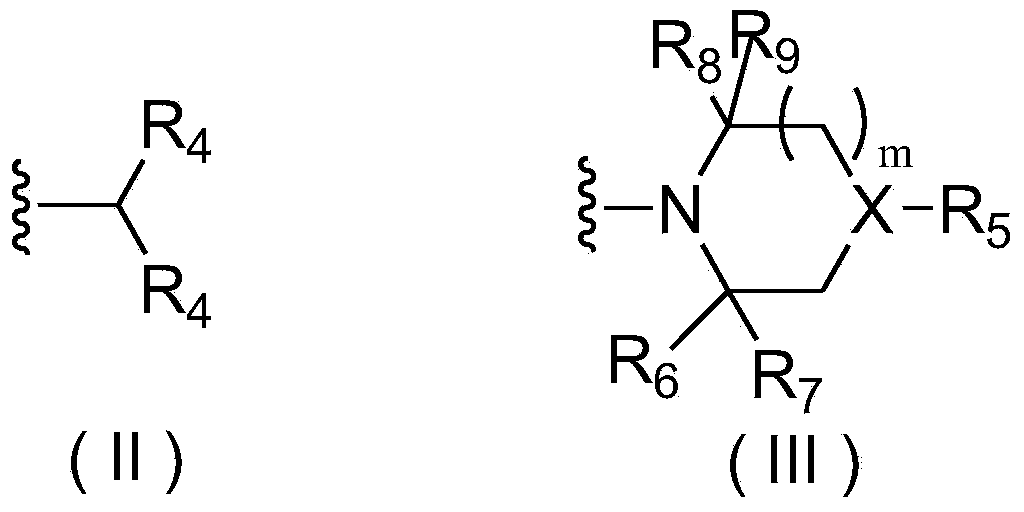
Pyrimidine derivatives and application thereof
A technology of pyrimidines and derivatives, which is applied to pyrimidine derivatives and its application in the treatment of pain diseases, can solve the problems of low response rate, large side effects of drugs, easy generation of drug resistance and addiction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1, 4-methyl-2-phenyl-6-(2-(morpholin-1-yl)ethoxy)pyrimidine (1)
[0091] Reaction 1
[0092]
[0093]1) Dissolve 12.0 g of benzamidine and 13.0 g of ethyl acetoacetate in 200 ml of methanol, stir in an ice bath, and cool to 0°C. Take 2.3 g of potassium tert-butoxide, add slowly, remove the ice bath after the addition, and stir to room temperature. Then heated to reflux and reacted for 6 hours. After the reaction was completed, 500 ml of distilled water was added, and the pH was adjusted to be neutral with 10% citric acid solution, and a white solid was precipitated. After filtering, the solid was washed twice with water, and recrystallized from 95% ethanol to obtain 11.3 g of white solid with a melting point of 218-221° C. and a yield of 60.7%.
[0094] 2) Take 9.3g of the first step product, 13.8g of anhydrous potassium carbonate, 18.8g of 1,2-dibromoethane, add 100ml of acetone, heat and reflux for 4 hours, cool to room temperature, filter, and evaporate...
Embodiment 2
[0097] Example 2, 4-methyl-2-phenyl-6-(3-(morpholin-1-yl)propoxy)pyrimidine (2)
[0098] Replace 1,2-dibromoethane with 1,3-dibromopropane, and prepare the target compound according to the method of Example 1.
[0099] 1 H NMR (600 MHz, CDCl 3 )δ8.45–8.41(m,2H),7.45–7.40(m,3H),6.44(s,1H),4.52(t,J=6.5Hz,2H),3.71(t,J=4.6Hz,4H ), 2.57–2.39(m,9H), 2.05–1.94(m,2H). MS(ESI) m / z 313.2([M+H] + )
Embodiment 3
[0100] Example 3, 4-methyl-2-phenyl-6-(4-(morpholin-1-yl)butoxy)pyrimidine (3)
[0101] Replace 1,2-dibromoethane with 1,4-dibromobutane, and prepare the target compound according to the method of Example 1.
[0102] 1 H NMR (600 MHz, CDCl 3 )δ8.45–8.41(m,2H),7.46–7.41(m,3H),6.44(s,1H),4.49(t,J=6.5Hz,2H),3.70(t,J=4.6Hz,4H ),2.48(s,3H),2.45–2.38(m,6H),1.88–1.80 (m,2H),1.71–1.63(m,2H).MS(ESI)m / z 328.2([M+H] + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com