Functionalized thieno-indole derivatives for the treatment of cancer
A technology of indole and compounds, applied in the field of functionalized thienoindole derivatives for the treatment of cancer, which can solve the problems of lack of tumor cell selectivity, non-optimal physical and chemical properties, and the impossibility of reaching tumor drug concentrations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
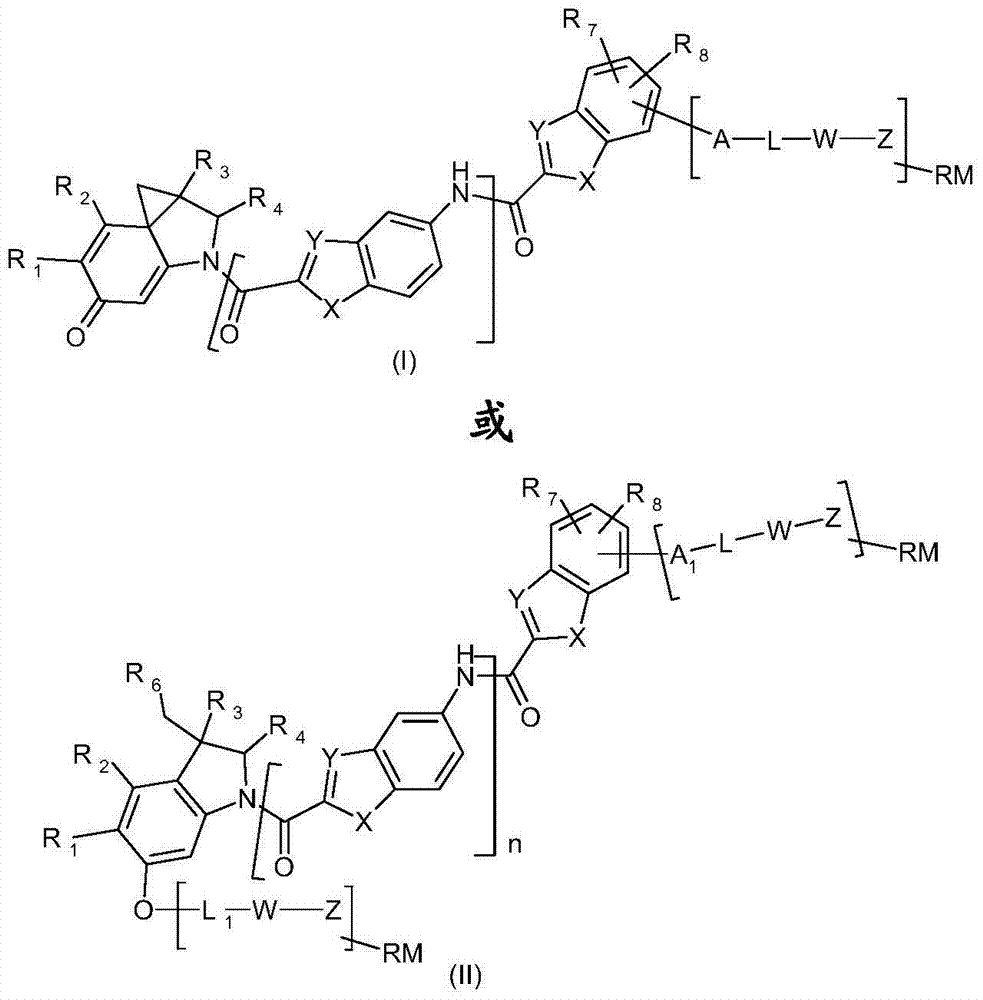
[0236] The present invention provides the preparation method of the compound of formula (I) or (II) defined above, it is characterized in that, described method comprises the following steps:
[0237] b) reacting a compound of formula (X) with a compound of formula (XIII),
[0238]
[0239] where X, Y, R 7 , R 8 and n are as defined above, and A 1 is A, wherein A is selected from OH, NH 2 and saturated groups of COOH,
[0240]
[0241] where R 16 Absent or hydrogen, halogen, -OH or -OR 17 , where R 17 is an activated moiety of a carboxyl group, such as an activated ester, or an activated -NH group, preferably p-toluenesulfonyl, and
[0242] L, W, Z and RM are as defined above, at least one of them is present;
[0243] c) reacting the resulting compound of formula (VIII) with a compound of formula (IX),
[0244]
[0245] Where X, Y, A 1 , R 7 , R 8 , L, W, Z, RM and n are as defined above,
[0246]
[0247] where R 14 is hydrogen or a protecting group,...
Embodiment 1
[0454] {2-[(2-{[(8S)-8-(chloromethyl)-4-hydroxy-1-methyl-7,8-dihydro-6H-thieno[3,2-e]indole -6-yl]carbonyl}-1H-indol-5-yl)carbamoyl]-1H-indol-5-yl}carbamate tert-butyl ester (XIV)
[0455] step c, step c', deprotection, step e
[0456]
[0457] step c
[0458](8S)-8-(Chloromethyl)-1-methyl-7,8-dihydro-6H-thieno[3,2-e]indol-4-ol prepared as reported in GB2344818 A solution of ((IX), 11.4 mg, 0.045 mmol) was dissolved in dry DMF (1 mL), washed with EDCI (35 mg, 4 eq.) and 5-[({5-[(tert-butoxycarbonyl)amino]- 1H-indol-2-yl}carbonyl)amino]-1H-indole-2-carboxylic acid (VIII) (29mg, 1.5eq.) as described in J.Med.Chem.2003, (46) The preparation reported on pages 634-637, the mixture was stirred at room temperature for 16 hours and then quenched by the addition of saturated aqueous NaCl. Isolation of the product was carried out by extraction with EtOAc (x4), followed by 2M aqueous HCl (x3), saturated Na 2 CO 3 The combined organic layers were washed with aqueous solution (x3)...
Embodiment 2
[0516] Coupling, deprotection, step e
[0517]
[0518] coupling
[0519]Preparation of (8S)-8-(chloromethyl)-6-({5-[(1H-indol-2-ylcarbonyl)amino]-1H-indol-2-yl}carbonyl)-1-methanol tert-butyl-7,8-dihydro-6H-thieno[3,2-e]indol-4-ylpiperazine-1,4-dicarboxylate
[0520]
[0521] N-(2-{[(8S)-8-(chloromethyl)-4-hydroxy-1-methyl-7,8-dihydro-6H-thieno[3,2-e]indole- 6-yl]carbonyl}-1H-indol-5-yl)-1H-indole-2-carboxamide (111 mg, 0.2 mmol) (prepared as reported in GB2344818) was dissolved in dry DCM (15 mL) and added to To this solution were added tert-butyl 4-(chlorocarbonyl)piperazine-1-carboxylate (100 mg, 0.4 mmol) and N,N-dimethylaminopyridine (55 mg, 0.45 mmol). The reaction mixture was stirred at room temperature under nitrogen atmosphere for 16 hours. The solvent was evaporated, the residue was dissolved in EtOAc, the resulting organic layer was washed with brine (x4), dried (Na 2 SO 4 ), concentrated under vacuum. The crude residue was purified by flash chromatogr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com