Non-natural difunctional saccharides, and preparing methods and applications thereof
A non-natural, dual-functional technology, applied in the field of chemical glycobiology, can solve problems such as the inability to separate and identify carbohydrate-binding proteins, and the inability to achieve high-throughput and real-time capture of carbohydrates and carbohydrate-binding proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of 9-AzSiaDAz (N-(4-diaziridine)valerylneuraminic acid)
[0048] SiaDAz (N-(4-diaziridine)pentanoylneuraminic acid) was used as the starting material to synthesize in three steps, and all reactions were carried out under dark conditions.
[0049] In the first step, add 1.38g SiaDAz (N-(4-diaziridine)pentanoylneuraminic acid), 50mL methanol and 0.5mL trifluoroacetic acid into a reaction vessel wrapped in aluminum foil, stir at room temperature for 12h, concentrate, and the residue Using dichloromethane and methanol (volume ratio 4:1) as the eluent, it was separated by chromatographic column to obtain 977 mg of 1-MeSiaDAz (N-(4-diaziridine)pentanoylneuraminic acid-1-methyl ester).
[0050] The characterization data of 1-MeSiaDAz (N-(4-diaziridine)pentanoylneuraminic acid-1-methyl ester) are as follows: 1 H NMR (500MHz,D 2 O)δ1.05(s,3H),1.73-1.78(m,2H),1.94(dd,J=12.0,12.5Hz,1H),2.22-2.27(m,2H),2.34(dd,J=4.5 ,13.0Hz,1H),3.62-3.67(m,2H),3.75-3.77(m,1...
Embodiment 2
[0055] Example 2 Synthesis of 9-AzSiaNAl (9-azido-N-(4-alkynyl)pentanoylneuraminic acid)
[0056] Using SiaNAl (N-(4-alkynyl)pentanoylneuraminic acid) as the starting material, it was synthesized through three-step reactions.
[0057] In the first step, 1.80g SiaNAl (N-(4-alkynyl)pentanoylneuraminic acid), 50ml methanol and 0.5ml trifluoroacetic acid were added to the reaction vessel, stirred at room temperature for 12h, concentrated, and the residue was dichloromethane and Methanol (volume ratio 4:1) was used as the eluent for chromatographic column separation to obtain 1.41 g of 1-MeSiaNAl (N-(4-alkynyl)pentanoylneuraminic acid-1-methyl ester).
[0058] The characterization data of 1-MeSiaNAl (N-(4-alkynyl)pentanoylneuraminic acid-1-methyl ester) are as follows: 1 H NMR (500MHz,D 2 O)δ1.94(dd, J=11.5,12.5Hz,1H),2.35(dd,J=5.0,13.0Hz,1H),2.43(br,1H),2.53-2.56(m,4H),3.62( dd,J=6.5,12.0Hz,1H),3.69(d,J=9.0Hz,1H),3.74-3.77(m,1H),3.85-3.88(m,4H),3.96-4.00(app t,1H ),4.08-4.12...
Embodiment 3
[0063] Example 3 Modification of unnatural bifunctional sugars to the cell surface
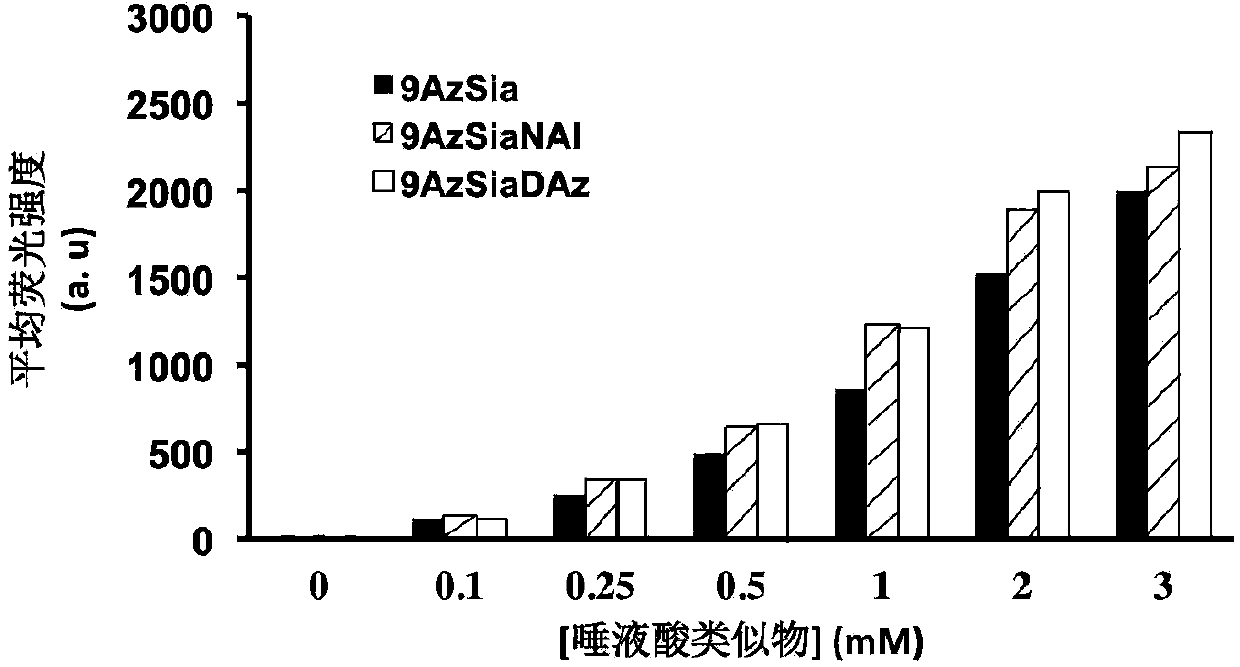
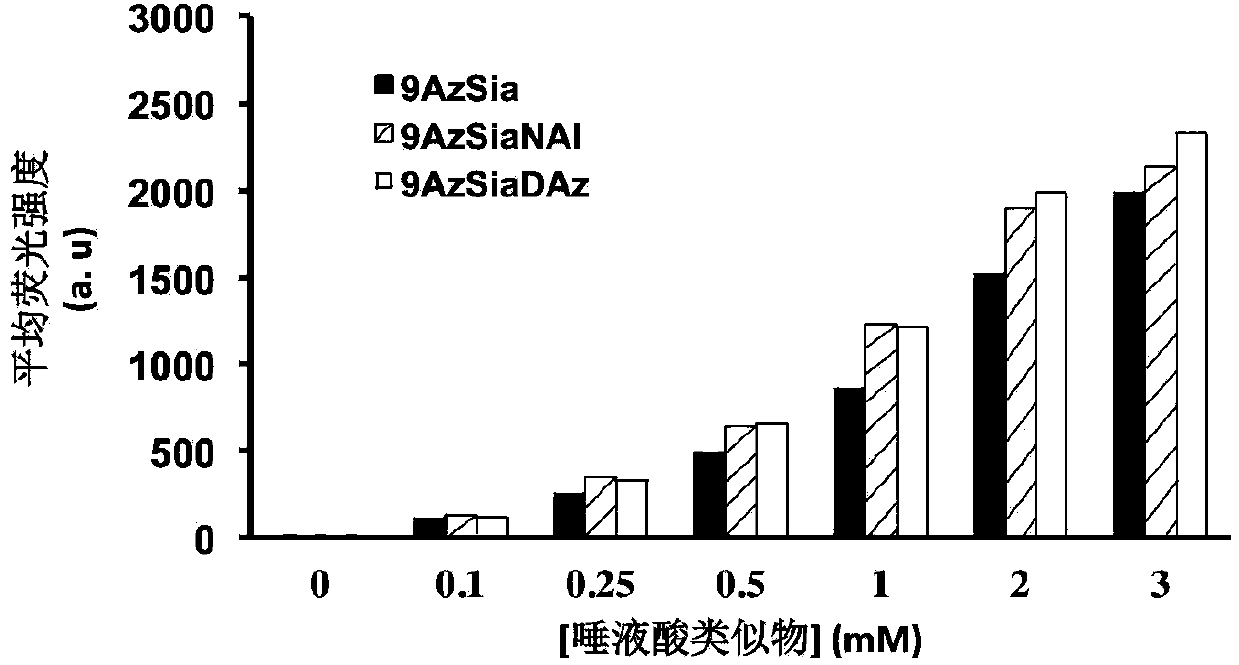
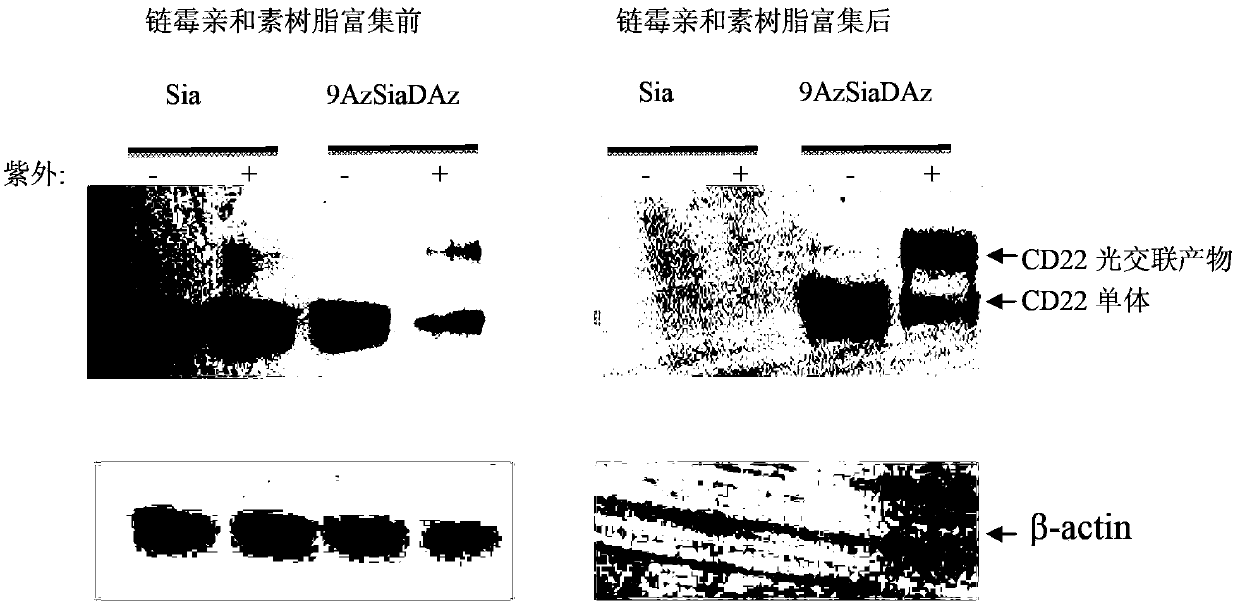
[0064] The non-natural bifunctional sugar 9-AzSiaNAl(9-azido-N-(4-alkynyl)pentanoyl neuron containing 0mM, 0.1mM, 0.25mM, 0.5mM, 1mM, 2mM and 3mM was used in concentration gradient expression experiments Azine) or 9-AzSiaDAz (9-azido-N-(4-diazido) valerylneuraminic acid) culture medium, with monofunctional unnatural sugar 9AzSia (9-azidoneuraminic acid ) as the control, the non-natural bifunctional sugar was expressed on the cell surface after 24 hours. In time expression experiments, 3 mM 9-AzSiaNAl (9-azido-N-(4-alkynyl)pentanoylneuraminic acid) and 9-AzSiaDAz (9-azido-N-(4-diaziridine ) valerylneuraminic acid) and 9-AzSia (9-azideneuraminic acid) were added to the cell culture medium at 0h, 4h, 8h, 12h, 16h, 20h and 24h, all the cells were collected and washed with PBS , let the cells perform a click reaction with sulfo-DBCO-Biotin to detect whether the cell surface contains azide group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com