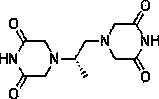
Dexrazoxane freeze-dried powder injection and preparation method thereof
A kind of technology of freeze-dried powder injection and dextrin, which is applied in the field of dextrin lyophilized powder for injection and preparation thereof, and can solve problems such as inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Composition of dextromethyridine freeze-dried powder injection:
[0040] 29.45g of dextranimine hydrochloride (equivalent to 25g of dextranimine);
[0041] Sodium acetate 35g;
[0042] Mannitol 20g.
[0043] Follow the steps below to prepare dextranimine freeze-dried powder injection:
[0044] (1) Dissolve sodium acetate in 400mL water for injection and stir until completely dissolved;
[0045] (2) Add mannitol into the solution prepared in (1), and stir until completely dissolved;
[0046] (3) Add dextranimine hydrochloride into the solution prepared in (2), and stir until completely dissolved;
[0047] (4) Adjust the pH to 2.5 with 0.1mol / L hydrochloric acid, and add water for injection to 500ml;
[0048] (5) Use 0.1% (W / V) activated carbon and stir for 15 minutes to absorb pyrogen;
[0049] (6) Rapidly filter with 0.45 μm and 0.22 μm microporous membranes, dispense into freeze-dried vials, 5 mL per vial, freeze-dry, stopper, and crimp the cap.
[0050] Freeze-...
Embodiment 2
[0053] Composition of dextromethyridine freeze-dried powder injection:
[0054] 29.45g of dextranimine hydrochloride (equivalent to 25g of dextranimine);
[0055] Sodium acetate 45g;
[0056] Mannitol 35g.
[0057] Follow the steps below to prepare dextranimine freeze-dried powder injection:
[0058] (1) Dissolve sodium acetate in 400ml water for injection and stir until completely dissolved;
[0059] (2) Add mannitol into the solution prepared in (1), and stir until completely dissolved;
[0060] (3) Add dextranimine hydrochloride into the solution prepared in (2), and stir until completely dissolved;
[0061] (4) Adjust the pH to 4.5 with 0.1mol / L hydrochloric acid, and add water for injection to the full amount;
[0062] (5) Use 0.1% (W / V) activated carbon and stir for 15 minutes to absorb pyrogen;
[0063](6) Rapidly filter with 0.45μm and 0.22βm microporous membranes, dispense into lyophilized vials, 5mL per vial, lyophilize, stopper, and crimp the cap.
[0064] Fr...
Embodiment 3
[0067] 29.45g of dextranimine hydrochloride (equivalent to 25g of dextranimine);
[0068] Sodium acetate 50g;
[0069] Mannitol 40g.
[0070] The specific operation process is the same as in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com