Anti-tumor fusion protein as well as encoding gene and application thereof
A technology of fusion protein and coding gene, which is applied in the direction of antineoplastic drugs, peptide/protein components, hybrid peptides, etc., can solve the problems of difficult acquisition and purification of the main product, small molecular weight, and no binding ability of tumor cells, so as to improve the anticancer Tumor curative effect, better anti-tumor effect, good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
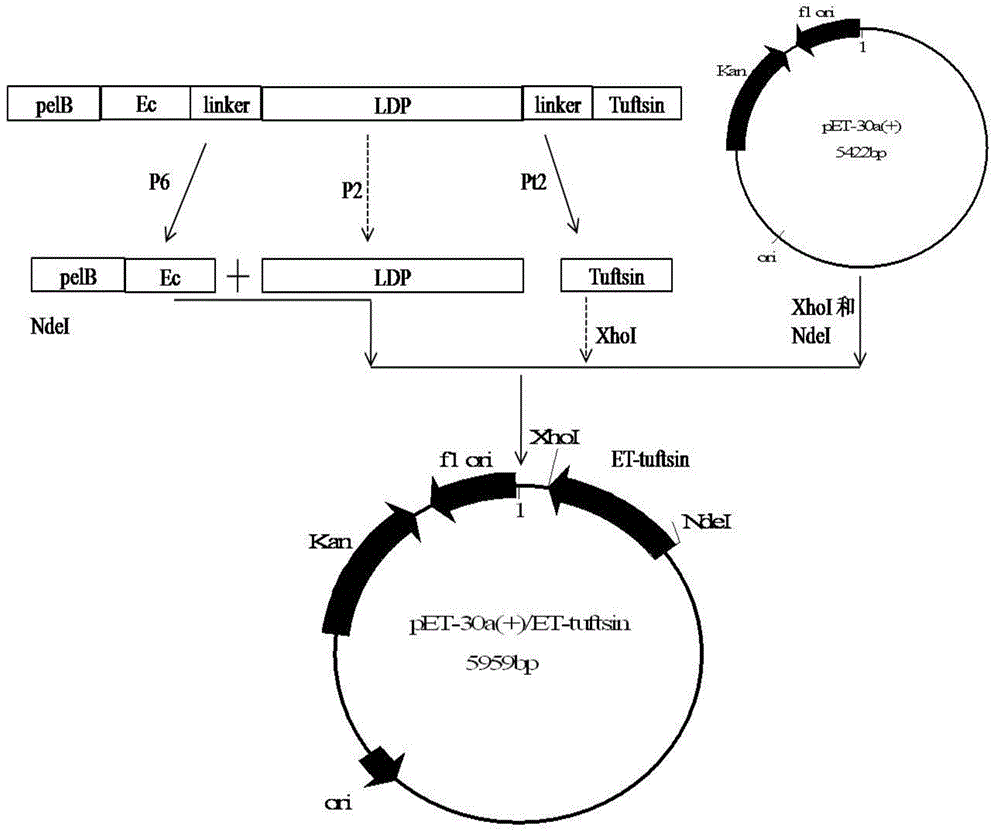
[0053] Example 1 Construction of recombinant expression vector pET30-ET-tuftsin
[0054] The recombinant plasmid pEL used in this example contains the EGFR oligopeptide ligand Ec gene and LDP gene. For the construction method and its map, please refer to Guo Xiaofang, "Construction of Oligopeptide Targeting Epidermal Growth Factor Receptor and Lidamycin Enhanced Fusion Protein and its antitumor activity", "Cancer", 2009, 28(6):561-568). Escherichia coli competent DH5α was a product of Beijing Quanshijin Biotechnology Co., Ltd., pET30a(+) was a product of Novagen (69909-3), and PCR primers were synthesized by Invigorate Shanghai Company.
[0055] Three primers are required to construct the recombinant vector pET30-ET-tuftsin:
[0056] E1 (SEQ ID NO:8): 5'-gc cat atg aaa tac ctg ctg ccg acc-3' (NdeI restriction site is underlined)
[0057] P2 (SEQ ID NO:9): 5'-gcc gaa ggt cag agc cac gtg-3'
[0058] T2 (SEQ ID NO: 10): 5'-gc ctc gag acg cgg ctt ggt tga acc gcc tcc acc...
Embodiment 2
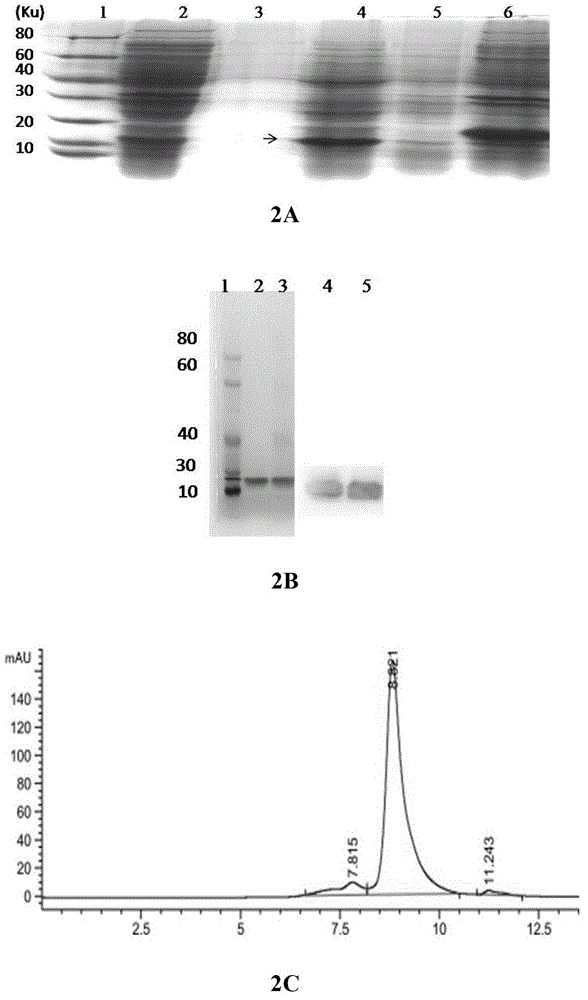
[0069] Example 2 Expression and Purification of Fusion Protein ET-tuftsin in Escherichia coli
[0070] Escherichia coli bacterial strain BL21star that the present invention uses TM (DE3) is a product of Novagen.
[0071] The recombinant expression vector pET30-ET-tuftsin constructed in Example 1 was transformed into Escherichia coli BL21, and a positive single clone was picked and inoculated in LB medium (kanamycin 50 μg / ml), and cultured overnight at 37°C. Sent for sequencing, and selected positive strains with correct sequencing and enzyme digestion results were stored at -80°C.
[0072]The positive single clone was inoculated into 5ml LB medium, cultured overnight at 37°C, when OD=1.0-2.0, added 1mmol / L IPTG, induced for 8h, and the culture supernatant, Periplasmic lumen, cytoplasmic soluble and insoluble fractions were analyzed. The fusion protein ET-tuftsin has a molecular weight of 16.3KD. It was found that ET-tuftsin protein was mainly localized in the periplasmic...
Embodiment 3
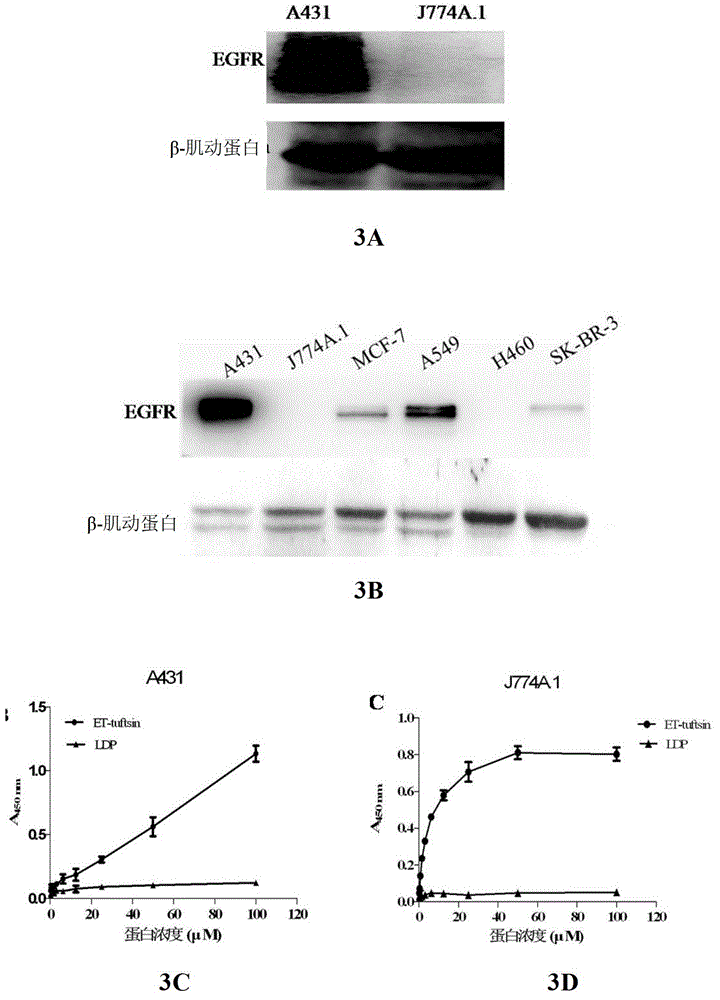
[0075] Example 3 Determination of cell binding activity of fusion protein ET-tuftsin in vitro
[0076] experiment one):
[0077] Human skin squamous cell carcinoma cell A431 and mouse macrophage J774A.1 in the logarithmic growth phase were washed twice with pre-cooled PBS, digested with trypsin, washed twice with PBS, and an appropriate amount of cell lysate (Beijing Baosai Biotechnology Company), lysed on ice for 10 minutes. Afterwards, centrifuge at 10,000 rpm for 30 minutes at 4°C, and collect the supernatant.
[0078] The fusion protein ET-tuftsin was quantified with the BCA kit, and 100 μg of the protein was mixed with an appropriate amount of 5× loading buffer, denatured in a boiling water bath for 5 minutes, and stored at -80°C for later use.
[0079] The expression level of EGFR in tumor cells was detected by Western Blot, and the results showed that A431 was a cell line with high expression of EGFR, while J774A.1 could not detect the expression of EGFR ( image 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com