Synthetic method of fondaparinux sodium intermediate
A technique for fondaparinux sodium and its synthetic method, which is applied in the fields of chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of long reaction steps and high reaction cost, and achieve reduction of production cost and simplification of synthesis route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
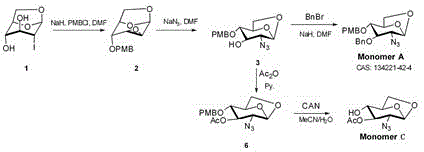
[0020] The preparation process of compound 6 is:
[0021] Compound 3 (40 g, 0.13 mol) was dissolved in pyridine (240 mL, 2.98 mol), and acetic anhydride (80 ml, 0.85 mol) was added. The reaction solution was stirred at 20°C for 1-3 hours, and concentrated to obtain compound 6 (43 g, 94.5%).
[0022] The reaction equation is: .
Embodiment 2
[0024] The preparation process of compound Monomer C ring is:
[0025] Compound 6 (42.5 g, 0.12 mol) was dissolved in acetonitrile (475 mL) and water (55 ml), cerium ammonium nitrate (166 g, 0.3 mol) was added at 0°C, and the reaction solution was reacted at 20°C for 1-3 Hours, after the reaction was completed, it was filtered, extracted, washed with aqueous sodium bicarbonate solution, dried, concentrated and passed through the column to obtain compound C ring (21.5 g, 78%).
[0026] The reaction equation is: .
[0027] 1 H NMR (400 MHz, CDCl 3 ) δ 5.42 (s, 1H), 4.88 – 4.81 (m, 1H), 4.58 (d, J = 5.4 Hz, 1H), 4.11 – 4.08 (d, J = 8 Hz ,1H), 3.80 (dd, J = 7.5, 5.8 Hz, 1H), 3.60 (s, 1H), 3.45 (s, 1H), 3.04 (s, 1H), 2.10 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com