Intermediate of fondaparinux sodium and preparation method for intermediate and fondaparinux sodium
An intermediate, sodium ion technology, applied in chemical instruments and methods, preparation of sugar derivatives, production of bulk chemicals, etc., can solve the difficulty of separation and purification of final products, problems with monitoring and separation, and poor reaction reproducibility, etc. problem, to achieve the effect of suitable for industrial scale production, easy monitoring and tracking, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
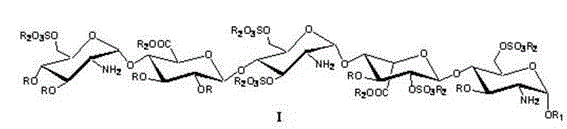
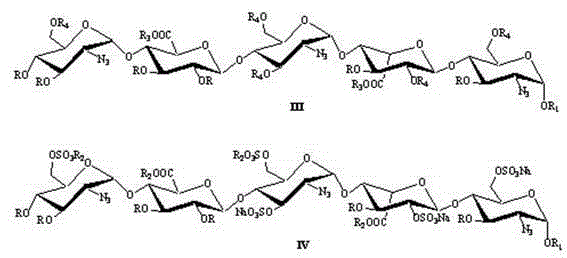
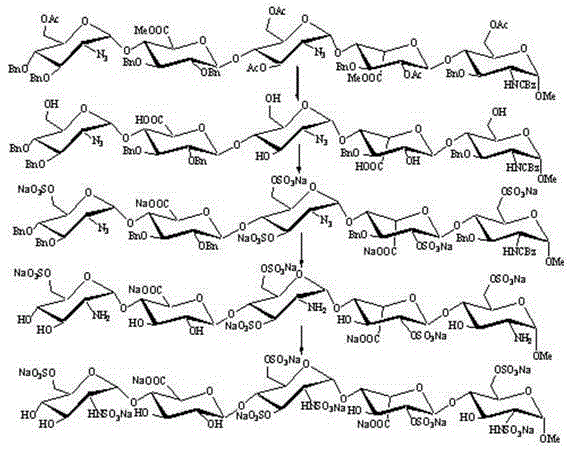
[0040] a. The intermediate compound 1 is: (2-azido-3,4-two- O -Benzyl-2-deoxy- α -D-glucopyranose)-(1→4)- O -(2,3-two- O -Benzyl- β -D-sodium glucopyranose)-(1→4)- O -(2-Azide-2-deoxy- α -D-glucopyranose)-(1→4)- O -(3- O -Benzyl- α -L-Pyran type iduronate sodium)-(1→4)-2-azido-3- O -Benzyl-2-deoxy- α - Preparation of D-glucopyranoside:
[0041]
[0042] Dissolve the fully protected pentasaccharide compound (2g, 5.4mmol) in 20mL tetrahydrofuran, add 1.0M sodium hydroxide aqueous solution 10mL dropwise at 0°C, stir at 4°C for 15 hours, HPLC shows that the reaction is complete, and then add 100mL ethyl acetate for extraction, and the organic phase with N a After washing with an aqueous solution of CI, dry it with anhydrous sodium sulfate, concentrate under reduced pressure, and then dissolve the foamy solid in water, and use an ion exchange column (Dowex 50 WX4 Na + ) through the column, with H 2 O / MeOH (1:1) was washed into the column, and the solvent was removed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com