Bithiophene-benzodi(benzoselenadiazole)-containing copolymer as well as preparation method and application thereof
A technology of benzoselenadiazole and bidithiophene is applied in the field of copolymers containing bidithiophene-benzodi and its preparation, which can solve the problem that the absorption spectrum and the solar spectrum cannot be well matched and reduce the photons in the solar spectrum. Absorption rate, low energy conversion efficiency of organic solar cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
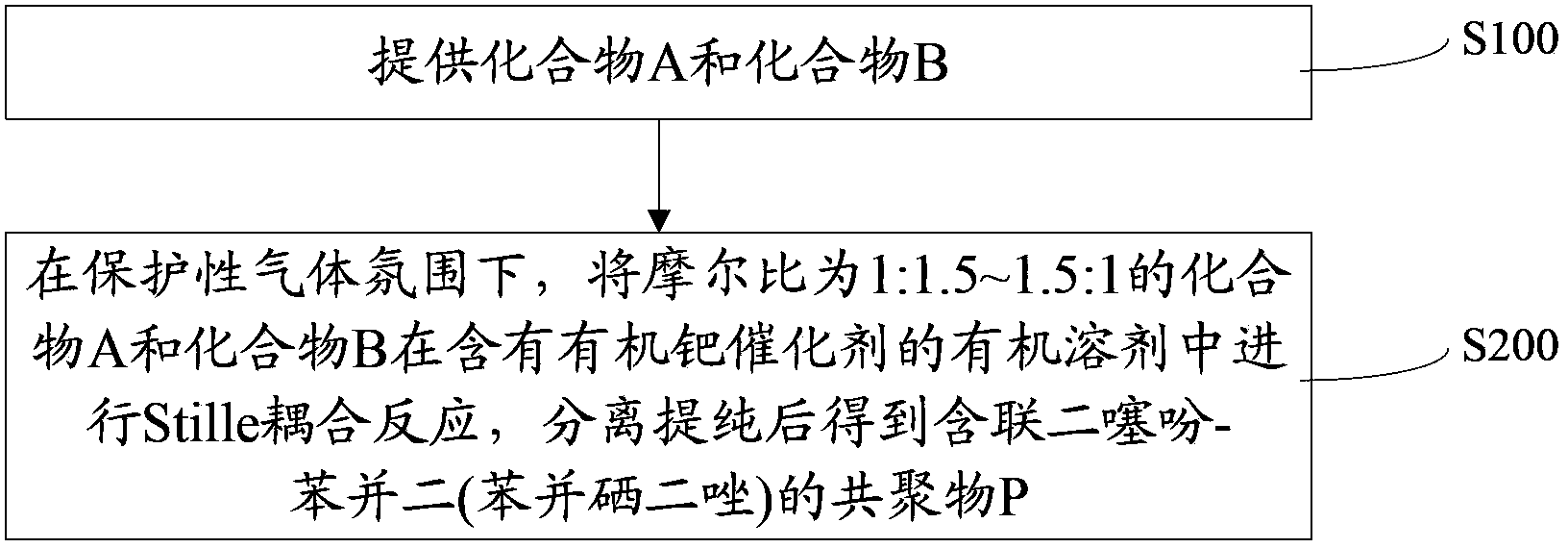
[0043] Such as figure 1 Shown, the preparation method of the copolymer containing bidithiophene-benzobis(benzoselenadiazole) of one embodiment, comprises the steps:
[0044] Step S100, providing compound A and compound B.
[0045] Compound A and Compound B have the following structural formulas respectively:
[0046] A is: B is: Among them, R 1 , R 2 , R 3 and R 4 for C 1 ~C 20 of alkyl.
[0047] The preparation of compound A comprises the following steps:
[0048] Step S111, providing compound C, n-butyllithium and tributyltin chloride.
[0049] The structural formula of compound C is where R 3 , R 4 for C 1 ~C 20 of alkyl.
[0050] Step S112, under a protective gas atmosphere, add compound C into tetrahydrofuran, and add n-butyllithium (CH 3 (CH 2 ) 3 Li), stirred for 1 hour to 2 hours, and added tributyltin chloride (SnBu 3 Cl), react at -78°C for 1 hour, warm up to room temperature and stir for 6 hours, add saturated aqueous sodium chloride solution...
Embodiment 1
[0107] This example discloses poly{4,4'-dioctyl-2,2'-bidithiophene-6,7-bis(3,7-dimethyl)octyl-benzo[2, 1-e:3,4-e]bis(benzoselenodiazole)} (copolymer P1 containing bidithiophene-benzobis(benzoselenodiazole)):
[0108] Among them, n=52.
[0109] The preparation process of the above-mentioned copolymer P1 containing bidithiophene-benzobis(benzoselenodiazole) is as follows:
[0110] 1.1. Preparation of compound 5-nitro-2,1,3 benzothiadiazole
[0111]
[0112] Add 2-amino-5-nitroaniline (22.95g, 0.15mol) and 100mL thionyl chloride (SOCl 2 ), stirred and slowly added 2mL of pyridine dropwise, heated and refluxed at 80-90°C for 24h, stopped the reaction, heated to 80°C and rotary evaporated excess SOCl 2 Finally, the reaction product was cooled to room temperature, poured into a large amount of water, stirred, filtered, washed with water, and then vacuum-dried to obtain 21.7 g of the product 5-nitro-2,1,3-benzothiadiazole with a yield of 80%.
[0113] 1.2. Preparation of com...
Embodiment 2
[0142] This example discloses poly{4,4'-dimethyl-2,2'-bidithiophene-6,7-bis(3,7-dimethyl)octyl-benzo[2, 1-e:3,4-e]bis(benzoselenoadiazole)} (copolymer P2 containing bidithiophene-benzobis(benzoselenoadiazole)):
[0143] Among them, n=22.
[0144] The preparation process of the above-mentioned copolymer P2 containing bidithiophene-benzobis(benzoselenodiazole) is as follows:
[0145] 2.1. Preparation of 4,4’-dimethyl-5,5’-bis(tributyltinyl)-2,2’-bithiophene
[0146]
[0147] Under the protection of nitrogen, add 4,4'-dimethyl-5,5'-dibromo-2,2'-bithiophene (3.52g, 0.01mol) into the three-necked flask, add 40mL of tetrahydrofuran solvent, Slowly inject n-butyllithium (8.6mL, 2.5M, 0.02mol) with a syringe at -78°C, continue stirring for 1.5h, inject tributyltin chloride (5.6 mL, 0.02mol), reacted for 1 hour, then warmed up to room temperature and stirred for 6 hours. Saturated aqueous sodium chloride (30 mL) was added to terminate the reaction, extracted with anhydrous eth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com