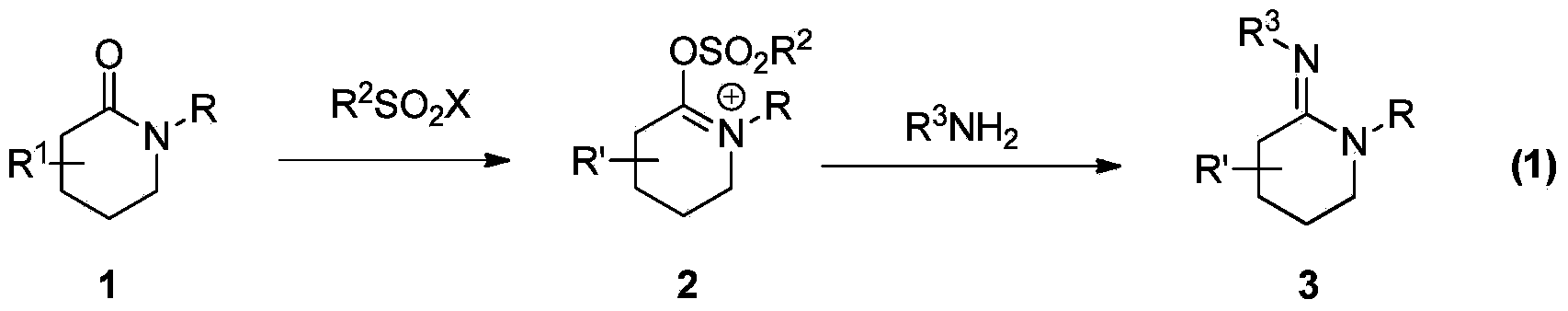
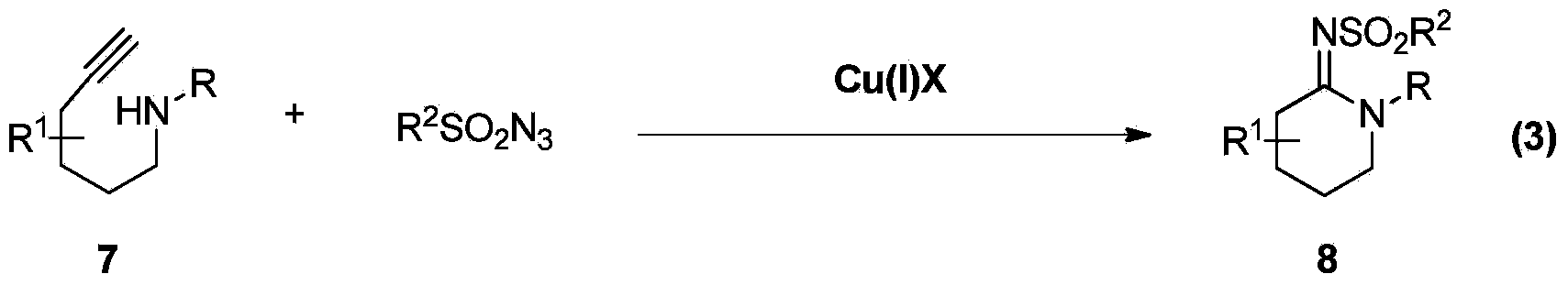Preparation method of multi-substituted cyclic amidine
A multi-substituted, cyclic amidine technology, applied in organic chemistry and other fields, can solve the problems of using dangerous reagents, narrow substrate range, severe reaction conditions, etc., and achieve the effect of simple operation, difficult preparation, and high stereo control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under nitrogen protection, 1.0 mmol of allylamine alkyne 9a (R = p-methoxyphenyl, R' = H, R" = H, n = 0), 1.0 mmol of p-toluenesulfonyl alkene Nitrogen and 5.0 moles of diisopropylethylamine are mixed in 5 ml of toluene, and 0.05 moles of copper 2-thiazole formate are added at 30 degrees, and the mixture is evenly mixed. After 24 hours, 2 ml of saturated ammonium chloride solution is added, and the stirring is continued. After 5 hours, wash with 5 ml of water and 5 ml of saturated brine successively, separate the organic phase and dry it with anhydrous sodium sulfate, filter and remove the organic solvent, add 1 g of 200 mesh silica gel and 6 ml of dichloromethane, mix well, and carefully distill out Solvent, put the remaining silica gel on a silica gel column, elute with ethyl acetate / petroleum ether, collect fractions, and obtain 93% cyclic amidine 10a. 10a: Yellow oil, 94%; 1 h NMR (400MHz, CDCl 3 )δ7.69(d, J=8.2Hz, 2H), 7.22(d, J=9.1Hz, 2H), 7.13(d, J=8.1Hz, 2H...
Embodiment 2
[0034] Under nitrogen protection, 1.0 mmol of allylamine alkyne 9b (R = benzyl, R' = phenyl, R" = hydrogen, n = 1), 1.0 mmol of p-toluenesulfonyl azide, 5.0 Mix moles of diisopropylethylamine in 5 ml of toluene, add 0.05 moles of copper 2-thiazole carboxylate at -20°C, stir and mix evenly, after 24 hours, add 2 ml of saturated ammonium chloride solution, and continue stirring for 5 hours , washed successively with 5 milliliters of water and 5 milliliters of saturated saline, separated the organic phase and dried it with anhydrous sodium sulfate, filtered and removed the organic solvent, added 1 gram of 200-mesh silica gel and 6 milliliters of dichloromethane and mixed evenly, carefully distilled off the solvent, The remaining silica gel was applied to a silica gel column, eluted with ethyl acetate / petroleum ether, and the fractions were collected to obtain 91% cyclic amidine 10b.
[0035] 10b: Yellow oil, 93%; 1 h NMR (400MHz, CDCl 3 )δ7.66(d, J=8.2Hz, 2H), 7.55(d, J=7.3...
Embodiment 3
[0037] Under nitrogen protection, 1.0 mmol of allylamine alkyne 9c (R = n-hexyl, R' = phenyl, R" = hydrogen, n = 0), 1.2 mmol of p-toluenesulfonyl azide, 5.0 One mole of diisopropylethylamine was mixed in 5 milliliters of tetrahydrofuran, and 0.05 moles of copper 2-thiazole formate was added at 60°C, stirred and mixed evenly. After 24 hours, 2 milliliters of saturated ammonium chloride solution was added, and the stirring was continued for 5 hours. Wash with 5 ml of water and 5 ml of saturated brine successively, separate the organic phase and dry it with anhydrous sodium sulfate, remove the organic solvent after filtration, add 1 g of 200 mesh silica gel and 6 ml of dichloromethane and mix well, carefully distill off the solvent, and the remaining Put the silica gel on a silica gel column, elute with ethyl acetate / petroleum ether, collect fractions to obtain 95% cyclic amidine 10c. 10c: Yellow oil, 86%; 1 H NMR (400MHz, CDCl 3 )δ7.80(d,J=8.0Hz,2H),7.22(d,J=7.9Hz,2H),5.80-5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com