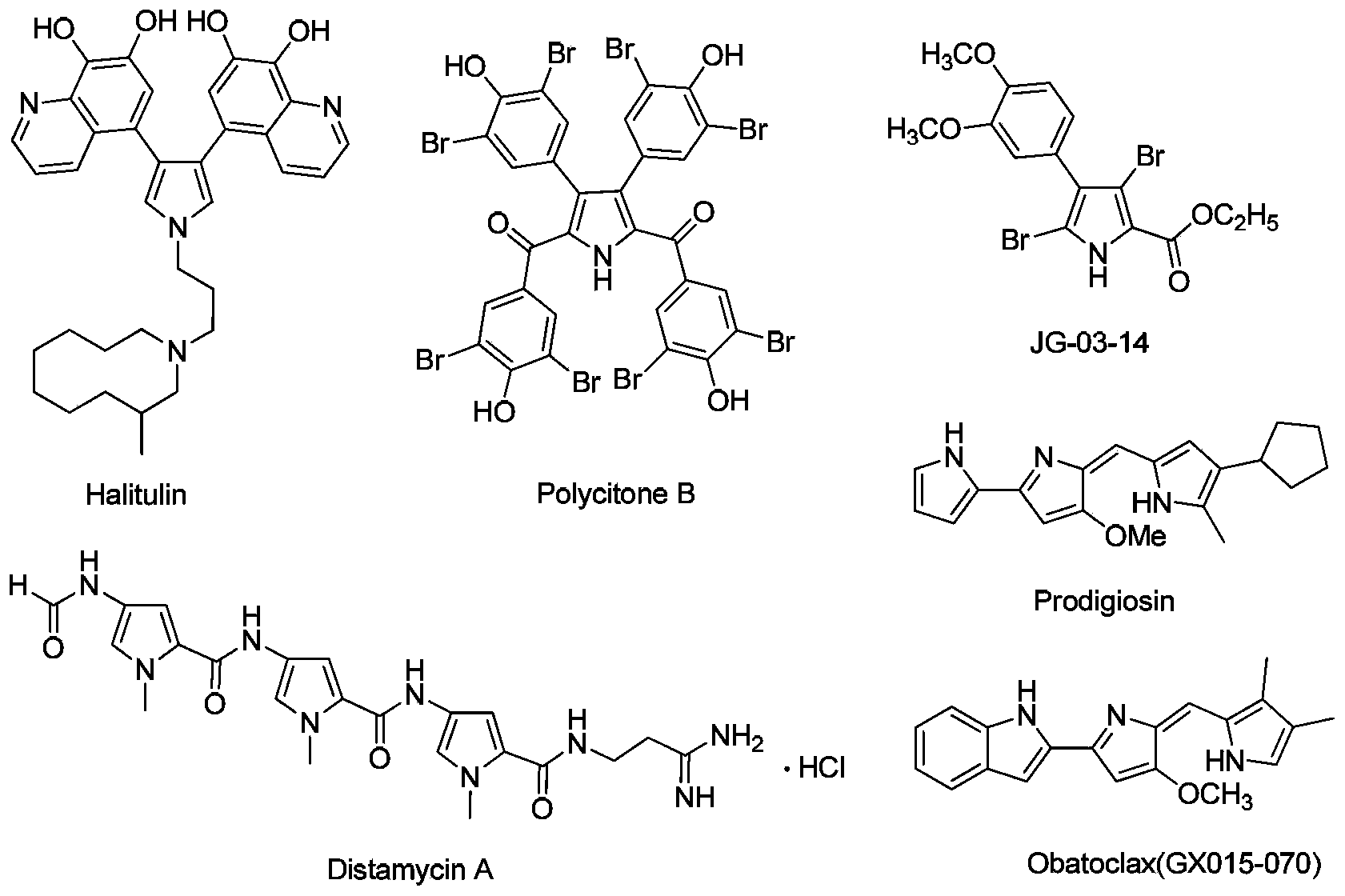
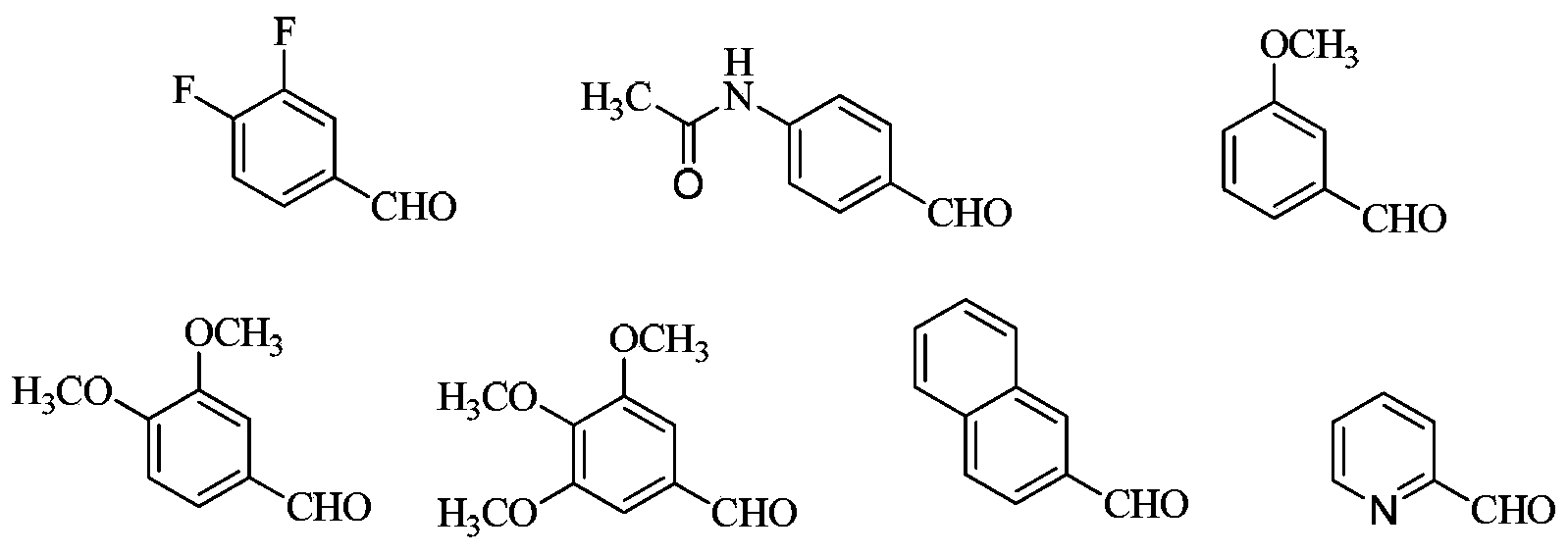
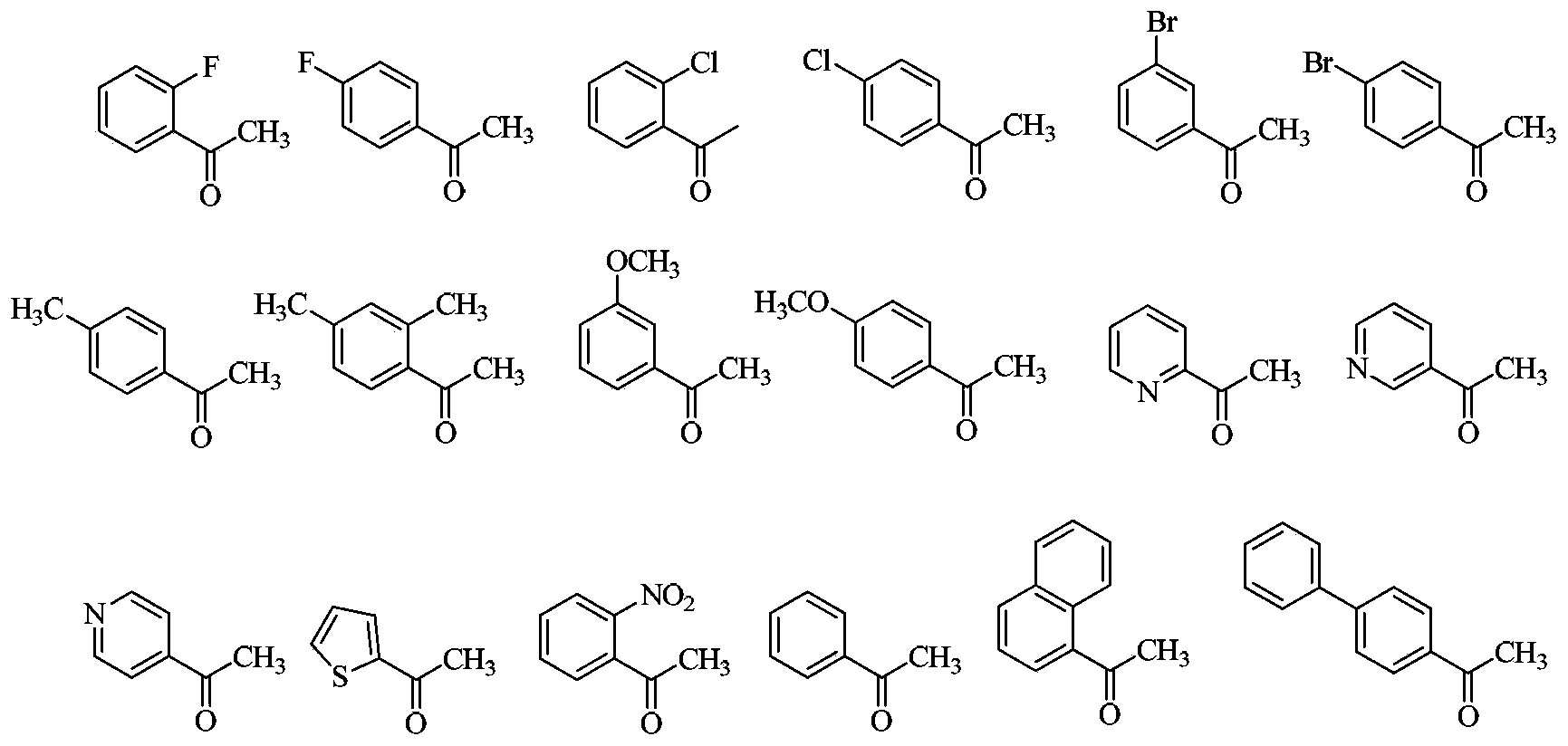
3,4-disubstituted pyrrole compound as well as preparation method and application thereof
A disubstituted pyrrole and compound technology, applied in 3 fields, can solve problems such as toxic and side effects, affecting normal dividing cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of (4-(3,4-Difluorophenyl)-1H-pyrrol-3-yl)(2-fluorophenyl)methanone:
[0056] Dissolve 1-(2-fluorophenyl)-3-(3,4-difluorophenyl)propenone (0.26g, 1.0mmol) and TosMIC (0.21g, 1.1mmol) in 10mL of dry THF or DMSO After stirring and cooling in an ice bath, t-BuOK (0.14g, 1.2mmol) was added slowly or in batches. After the reactants were fully stirred and contacted, they could react at room temperature. The reaction was stopped after 2h. The excess t-BuOK was quenched with ice-saturated aqueous ammonium chloride solution. The reaction solution was extracted three times with ethyl acetate (75mL, 25mL×3). The organic layers were combined and washed once with saturated brine. Sodium sulfate is allowed to stand to dry. The solvent was evaporated under reduced pressure, and silica gel column chromatography (petroleum ether: ethyl acetate = 1:2) gave white (4-(3,4-difluorophenyl)-1H-pyrrol-3-yl)(phenyl) ) Methyl ketone solid (0.19 g, 0.64 mmol), yield 69.4%.
[0057] The ex...
Embodiment 2
[0060] Synthesis of (4-(3,4-Difluorophenyl)-1H-pyrrol-3-yl)(4-fluorophenyl)methanone
[0061] The preparation method is the same as that of Example 1, and the raw materials are 1-(4-fluorophenyl)-3-(3,4-difluorophenyl)propenone and TosMIC.
[0062] The product is a yellowish solid with a yield of 83.7%.
[0063] The experimental data of the product is as follows:
[0064] 1H NMR (400MHz, DMSO) δ (ppm): 11.77 (s, 1H, NH), 7.76 (dd, J1 = 6.0 Hz, J2 = 8.4 Hz, 2H, -CO-Ar-H), 7.48 (ddd, J1 =1.6Hz, J2=8.0Hz, J3=12Hz, 1H, Ar-H), 7.36-7.26 (m, 4H, Ar-H+-CO-Ar-H+pyrrole-H), 7.25-7.20 (m, 1H) , Ar-H), 7.19 (s, 1H, pyrrole-H); 13C NMR (100MHz, DMSO-d6) δ (ppm): 188.79, 165.29, 162.81, 150.08, 149.95, 149.10, 148.97, 147.66, 147.54, 146.68 , 146.55, 136.41, 132.77, 131.66, 131.57, 128.57, 124.86, 123.30, 120.41, 120.22, 117.12, 116.94, 116.64, 116.48, 115.19, 114.98.
Embodiment 3
[0066] Synthesis of (4-(3,4-Difluorophenyl)-1H-pyrrol-3-yl)(2-chlorophenyl)methanone
[0067] The preparation method is the same as in Example 1. The raw materials are 1-(2-chlorophenyl)-3-(3,4-difluorophenyl)propenone and TosMIC.
[0068] The product is a yellowish solid with a yield of 76.2%.
[0069] The experimental data of the product is as follows:
[0070] 1H NMR (400MHz, DMSO) δ (ppm): 11.81 (s, 1H, NH), 7.59 (dd, J1 = 8.0 Hz, J2 = 12.8 Hz, 1H, -CO-Ar-H), 7.53-7.44 (m , 3H, -CO-Ar-H), 7.42 (d, J=6.8 Hz, 1H, Ar-H), 7.39-7.33 (m, 2H, Ar-H), 7.16 (s, 1H, pyrrole-H) , 7.02 (s, 1H, pyrrole-H); 13CNMR (100MHz, DMSO-d6) δ (ppm): 188.48, 149.98, 149.85, 149.30, 149.18, 147.56, 147.43, 146.88, 146.75, 140.52, 132.43, 130.77, 130.59 , 129.53, 128.57, 126.80, 125.17, 122.92, 121.30, 120.89, 117.43, 117.25, 116.59, 116.42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com