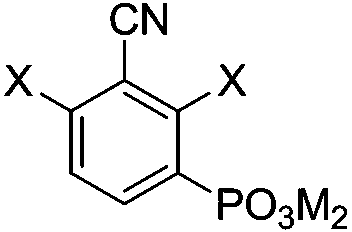
Preparation methods of 3-nitrile-2,4-dihalogeno phenylphosphonic acid and salts thereof
A technology of dihalophenylphosphonic acid and dihalophenylphosphonate is applied in the technical field of polymer materials, and can solve the problems of easy deformation, narrow pH range, increasing water treatment process technology and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
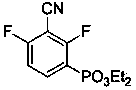
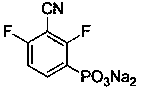
[0041] Preparation of sodium 3-cyano-2,4-difluorophenylphosphonate:
[0042] (1) Preparation of 3-bromo-2,6-difluorobenzonitrile:
[0043]
[0044] Take a 2 L four-neck flask, add 600 mL of 60% sulfuric acid, add 139.0 g (1.0 mol) of 2,6-difluorobenzonitrile under electric stirring, and 2 Under protection, control the reaction temperature below 35°C, add 250.5 g of KBrO in 10 batches 3 (1.5 mol), monitored by TLC, reacted for 5-6 days, stopped the reaction after the raw material point disappeared, prepared 800 mL of 20% ice-salt solution, and poured the reaction solution into ice-salt water. After the product settles, quickly carry out suction filtration to obtain an orange crude product, which is washed with CCl 4 Dissolve the crude product with 5% NaHSO 3 solution, saturated Na 2 CO 3 Wash the solution and water until the solution is neutral, dry it with anhydrous sodium sulfate, filter the solution, and steam in a water bath at 60°C to obtain a light yellow liquid, ...
Embodiment 2
[0052] Preparation of sodium 3-cyano-2,4-dichlorophenylphosphonate:
[0053] (1) Preparation of 3-bromo-2,6-dichlorobenzonitrile:
[0054]
[0055] Take a 2 L four-neck flask, add 600 mL of 60% sulfuric acid, add 172.0 g (1.0 mol) of 2,6-dichlorobenzonitrile under electric stirring, and 2 Under protection, control the reaction temperature below 35°C, add 250.5 g of KBrO in 10 batches 3 (1.5 mol), monitored by TLC, reacted for 5-6 days, stopped the reaction after the raw material point disappeared, prepared 800 mL of 20% ice-salt solution, and poured the reaction solution into ice-salt water. After the product settles, quickly carry out suction filtration to obtain an orange crude product, which is washed with CCl 4 Dissolve the crude product with 5% NaHSO 3 solution, saturated Na 2 CO 3 Wash the solution and water until the solution is neutral, dry it with anhydrous sodium sulfate, filter the solution, and steam in a water bath at 60°C to obtain a light yellow liquid, ...
Embodiment 3
[0063] Preparation of lithium 3-cyano-2,4-difluorophenylphosphonate:
[0064]
[0065] Mix diethyl 3-cyano-2,4-difluorophenylphosphonate with a certain amount of concentrated hydrochloric acid, raise the temperature to 110°C, heat to reflux for 24 h, and extract the reaction solution with ethyl acetate several times after cooling. Combine the organic phases, overnight with anhydrous magnesium sulfate, filter, and rotary evaporate to obtain the crude product of 3-cyano-2,4-difluorophenylphosphonic acid, dissolve the crude product in water, adjust the pH value to 7-8 with lithium hydroxide solution, and evaporate to dryness water, the obtained solid was dissolved in methanol, the insoluble matter was filtered off, methanol was spin-dried, and the obtained solid was recrystallized from water and ethanol to obtain lithium 3-cyano-2,4-difluorophenylphosphonate with a yield of 94%. 1 H-NMR (D 2 O): δ = 7.96-7.87 (m, 1 H), 7.08 (t, J = 8.8 Hz, 1 H) ppm; 31 P-NMR (D 2 O): δ = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com