Detection method for preparing puerperal blood-stasis dispersing capsule
A detection method and capsule technology, which are applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of incomplete preparation methods, unable to meet the needs of actual production, and difficult to achieve therapeutic effects, and achieve simple detection methods and controlled production. Cost, effect of precise control of quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
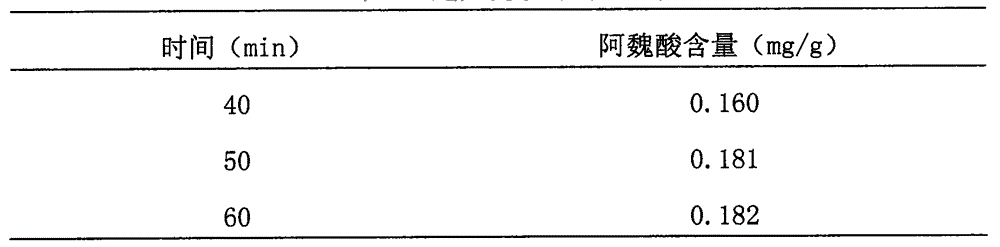
[0026] Motherwort 1248g Angelica 156g Chuanxiong 178g Paojiang 78g
[0027] Preparation:
[0028] For the above four flavors, take 20g each of motherwort, angelica, and chuanxiong, and grind them into fine powder, and set aside. Add 10 times the amount of leftover angelica, chuanxiong, and Paojiang to distill and extract for 3 hours, collect volatile oil, add 10 times the amount of dregs and remaining motherwort, respectively, Decoct with 8 times the amount of water twice, each time for 2 hours, and the second time for 1 hour, filter, combine the filtrates, concentrate to a thick paste with a relative density of 1.35 (50°C), add the above fine powder, mix well, and dry. Make granules, then dry, add volatile oils such as angelica, mix well, pack into capsules, and make 1000 granules, and you get it.
[0029] Detection method:
[0030] (1) Take 10 capsules of this product, pour out the contents, grind finely, add 10ml of absolute ethanol for ultrasonic treatment for 20 minutes...
experiment example 1
[0038] Experimental Example 1: TLC Identification of Chinese Angelica and Chuanxiong
[0039]Angelica and Chuanxiong were used as positive controls (provided by China Institute for the Control of Pharmaceutical and Biological Products, batch number 120927-200310, 120918-200406), and n-hexane-ethyl acetate (9:1) was used as the expansion point, and the spots after expansion were viewed at 365 nm , the fluorescent spots are round and round, and the separation effect is good. In addition, take other medicinal materials that do not contain angelica and chuanxiong in the prescription to make a negative sample according to the preparation method, and then make a negative sample solution according to the preparation method of the test solution. After three batches of samples and negative control tests, negative without interference.
[0040] Which attached figure 1 Among them, 1. Three batches of samples of Angelica as reference medicinal material 2. Three batches of samples of Chu...
experiment example 2
[0041] Experimental example 2: TLC identification of motherwort medicinal materials
[0042] With stachydrine hydrochloride reference substance (National Institute for the Control of Pharmaceutical and Biological Products, batch number 110712-200306) and motherwort reference medicinal material (National Institute for the Control of Pharmaceutical and Biological Products, batch number 1120912-200306) as positive controls, once compared I: n-butanol- Developing agents such as hydrochloric acid-water (4:1:0.5), II: acetone-absolute ethanol-hydrochloric acid (10:6:1), among which the spots after developing with developing agent II are rounded and the separation effect is good. Take other medicinal materials that do not contain motherwort in the prescription to make a negative sample according to the preparation method, and then make a negative sample solution according to the preparation method of the test solution. After three batches of samples and negative control tests, negati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com