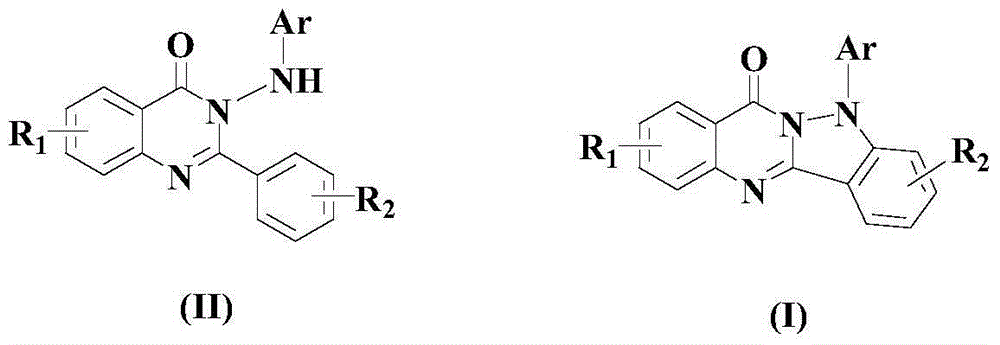
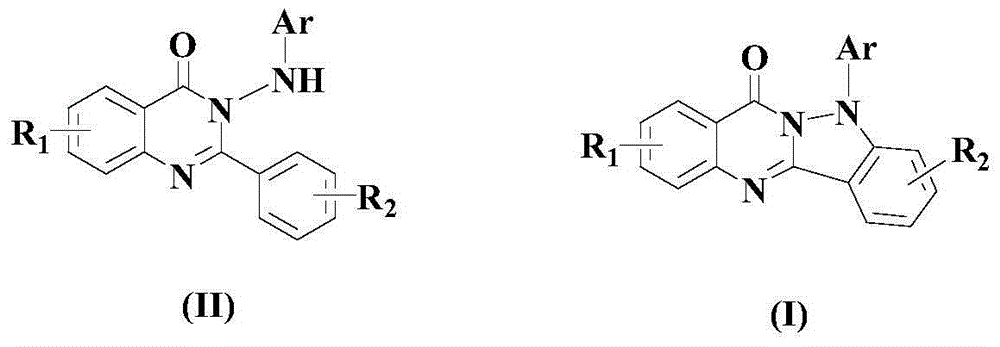
Method for synthesizing quinazolino indazole derivatives under acidic condition
A synthetic method, quinazolinone technology, applied in the field of organic chemical synthesis, can solve the problem of many components, and achieve the effects of high product yield, high purity, good scientific research value and application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: 5-phenylindazol[3,2-b]quinazolin-7(5H)-one
[0050]
[0051] 50ml solvent acetic acid is added in the reaction vessel, then add 10mmol (II) compound, 0.5mmol Pd(OAc) 2 , 30mmol of silver acetate, under the stirring of the stirring bar, heat up to 80 ℃ and seal the reaction for 48 hours.
[0052] After the reaction finished, the reaction mixture was cooled to room temperature, transferred to a separatory funnel, added ethyl acetate that was 3-5 times the volume of the mixture, and then added a 20% aqueous sodium hydroxide solution to adjust the pH value of the system to Alkaline, liquid separation, the organic layer was washed 1-3 times with saturated brine, and then rotatively evaporated, and the residue was passed through a 300-400 mesh silica gel column with ethyl acetate / petroleum ether as the eluent, in which ethyl acetate and petroleum The volume ratio of ether was 1:5, and the target product formula (I) compound 5-phenylindazol[3,2-b]quinazolin-7(5H...
Embodiment 2
[0056] Example 2: 5-p-tolyl indazol[3,2-b]quinazolin-7(5H)-one
[0057]
[0058] Add 50ml of solvent acetic acid to the reaction vessel, then add 10mmol of (II) compound, 1mmol of Pd(OAc) 2 , 40mmol silver acetate, under the stirring of the stirring bar, heat up to 90 ℃ and seal the reaction for 40 hours.
[0059] After the reaction finished, the reaction mixture was cooled to room temperature, transferred to a separatory funnel, added ethyl acetate that was 3-5 times the volume of the mixture, and then added a 20% aqueous sodium hydroxide solution to adjust the pH value of the system to Alkaline, liquid separation, the organic layer was washed 1-3 times with saturated brine, and then rotatively evaporated, and the residue was passed through a 300-400 mesh silica gel column with ethyl acetate / petroleum ether as the eluent, in which ethyl acetate and petroleum The volume ratio of ether was 1:10, and the target product formula (I) compound 5-p-tolyl indazol[3,2-b]quinazolin-...
Embodiment 3
[0063] Example 3: 5-p-trifluorotolyl indazol[3,2-b]quinazolin-7(5H)-one
[0064]
[0065] 50ml solvent acetic acid is added in the reaction vessel, then add 10mmol (II) compound, 1.5mmol Pd(OAc) 2 , 50mmol of silver acetate, under the stirring of the stirring bar, heat up to 100 ℃ and seal the reaction for 30 hours.
[0066] After the reaction finished, the reaction mixture was cooled to room temperature, transferred to a separatory funnel, added ethyl acetate that was 3-5 times the volume of the mixture, and then added a 20% aqueous sodium hydroxide solution to adjust the pH value of the system to Alkaline, liquid separation, the organic layer was washed 1-3 times with saturated brine, and then rotatively evaporated, and the residue was passed through a 300-400 mesh silica gel column with ethyl acetate / petroleum ether as the eluent, in which ethyl acetate and petroleum The volume ratio of ether was 1:15, and the target product formula (I) compound 5-p-trifluorophenylindaz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com