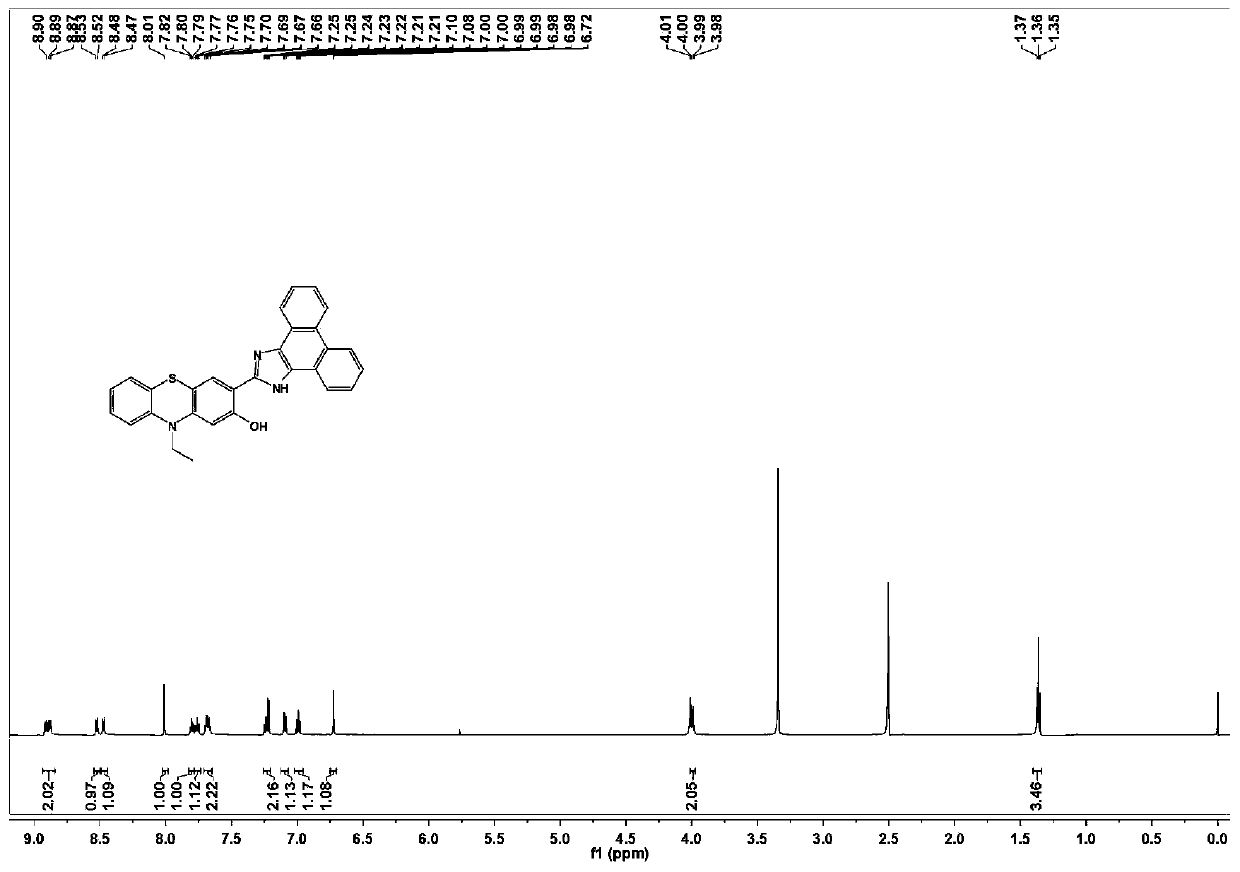
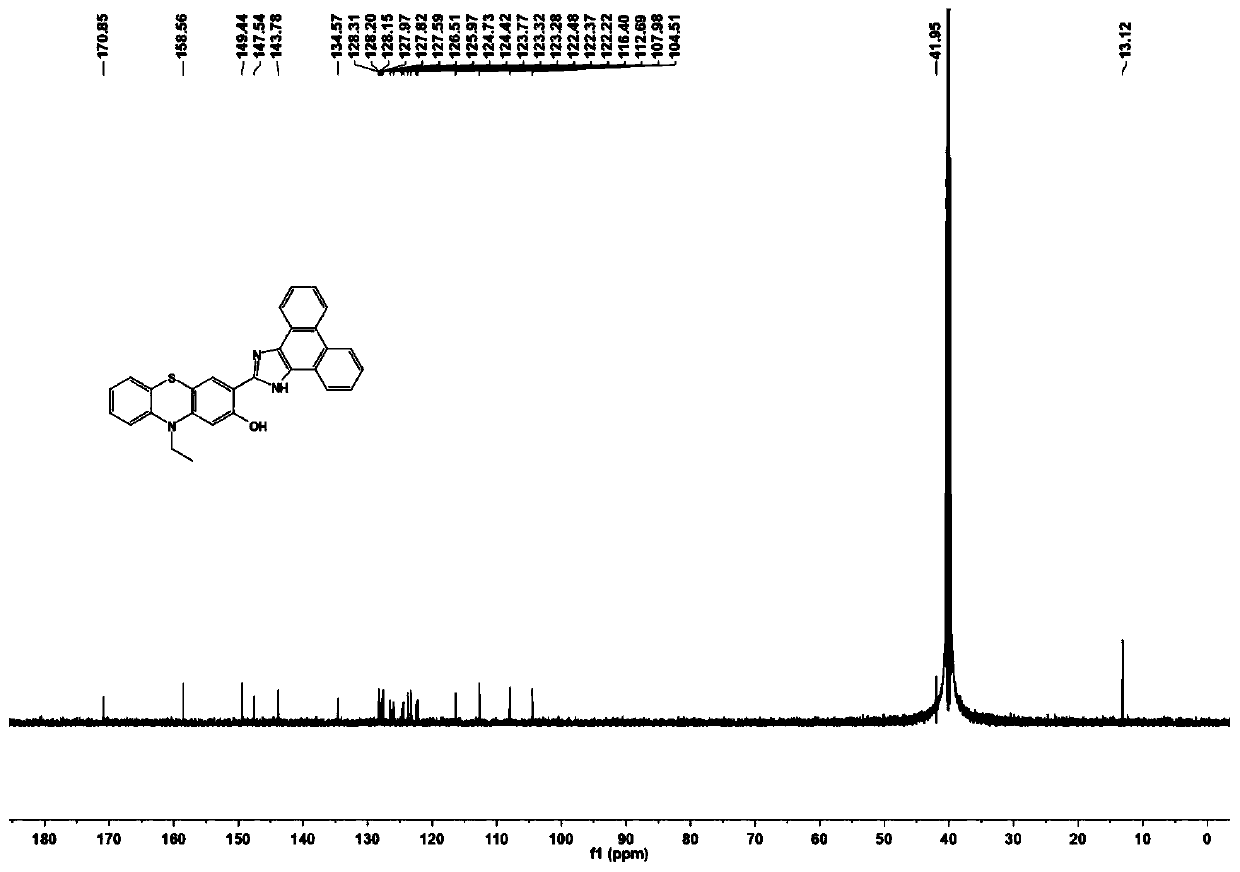
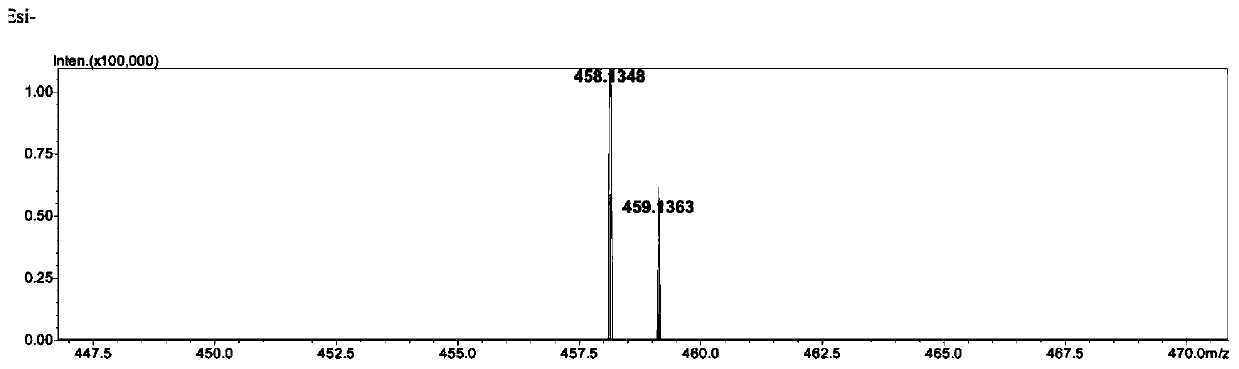
Phenothiazine compound and preparation method and application thereof
A compound and phenothiazine technology, applied in the field of functional compound synthesis, can solve the problems of insufficient phosgene selectivity, complicated fluorescent probe synthesis, low yield, etc., achieving stable structure, high preparation yield, and improved reaction selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1 intermediate 1
[0035]
[0036] Iodoethane (3.9g, 25mmol) and 2-methoxyphenothiazine (2.29g, 10mmol) were dissolved in 20mL of dimethyl sulfoxide, then NaOH (0.8g, 20mmol) was added, and the temperature was raised to 65°C for 15 Hour. After the reaction was completed, cool to room temperature, add 300mL of water, add dichloromethane (50mL×2) for extraction, wash with saturated brine (50mL×2), dry over anhydrous sodium sulfate, then remove the solvent under reduced pressure, and use the obtained residue with Separation on a 200-300 mesh silica gel column gave a white solid (2.3 g, 89.5%).
[0037] 1 H NMR (600MHz, DMSO-d 6 )δ7.14 (ddd, J=8.4, 7.2, 1.4Hz, 1H), 7.08 (dd, J=7.6, 1.7Hz, 1H), 7.02–6.94 (m, 2H), 6.92–6.86 (m, 1H) ,6.51(d,J=7.6Hz,2H),3.87(q,J=6.9Hz,2H),3.70(s,3H),1.25(t,J=6.9Hz,3H).
Embodiment 2
[0038] The synthesis of embodiment 2 compound 2
[0039]
[0040] Under nitrogen protection, 1.7mL DMF was added dropwise in phosphorus oxychloride (1mL) under ice bath, after stirring for 15 minutes, DMF solution (7.5mL) of Intermediate 1 (2.57g, 10mmol) was added dropwise to in the above reaction solution. After the dropwise addition, the temperature was raised to 60°C to continue the reaction for 4 hours, then the reaction solution was poured into ice water, stirred for a while, and saturated NaHCO 3 The solution was neutralized to neutral pH, then dichloromethane (50mL×3) was added for extraction, washed with saturated brine (50mL×2), dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure, and the resulting residue was washed with 200-300 Separation on a silica gel column to obtain a yellow solid (2.48g, 87%).
[0041] 1 H NMR (600MHz, Chloroform-d) δ10.19(s,1H),7.51(s,1H),7.13(t,J=7.6Hz,1H),7.09(dd,J=7.6,1.5Hz,1H) ,6.93(t,J=7.3Hz,1H...
Embodiment 3
[0042] The synthesis of embodiment 3 intermediate 3
[0043]
[0044] Aluminum trichloride (1.98 g, 10 mmol) was added to anhydrous dichloromethane (15 mL), and then a solution of Intermediate 3 (1.42 g, 5 mmol) in anhydrous dichloromethane (5 mL) was added dropwise thereto. After the dropwise addition, the reaction was continued at 20° C. for 12 hours, and then hydrochloric acid solution (2M, 6 mL) was added to quench the reaction. Ethyl acetate (20mL×3) was added for extraction, washed with saturated brine (30mL×2), dried over anhydrous sodium sulfate, and then the solvent was removed under reduced pressure, and the resulting residue was separated with a 200-300 mesh silica gel column to obtain a yellow solid ( 1.26g, 92.6%).
[0045]1 H NMR (600MHz, Chloroform-d) δ11.39 (s, 1H), 9.60 (d, J = 0.6Hz, 1H), 7.21–7.11 (m, 2H), 7.10 (dd, J = 7.7, 1.7Hz, 1H), 6.97(td, J=7.5, 1.3Hz, 1H), 6.92(dd, J=8.3, 1.3Hz, 1H), 6.39(s, 1H), 3.95(q, J=7.2Hz, 2H), 1.45(t,J=7.1Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com