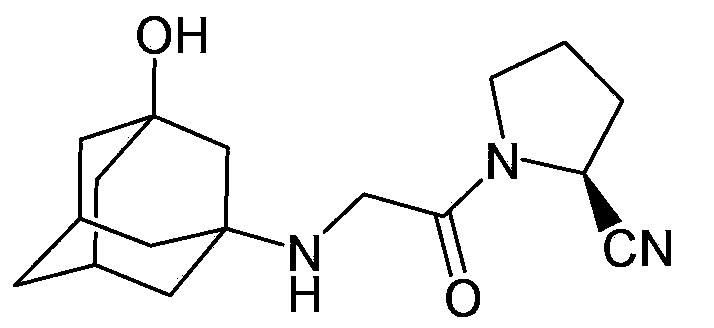
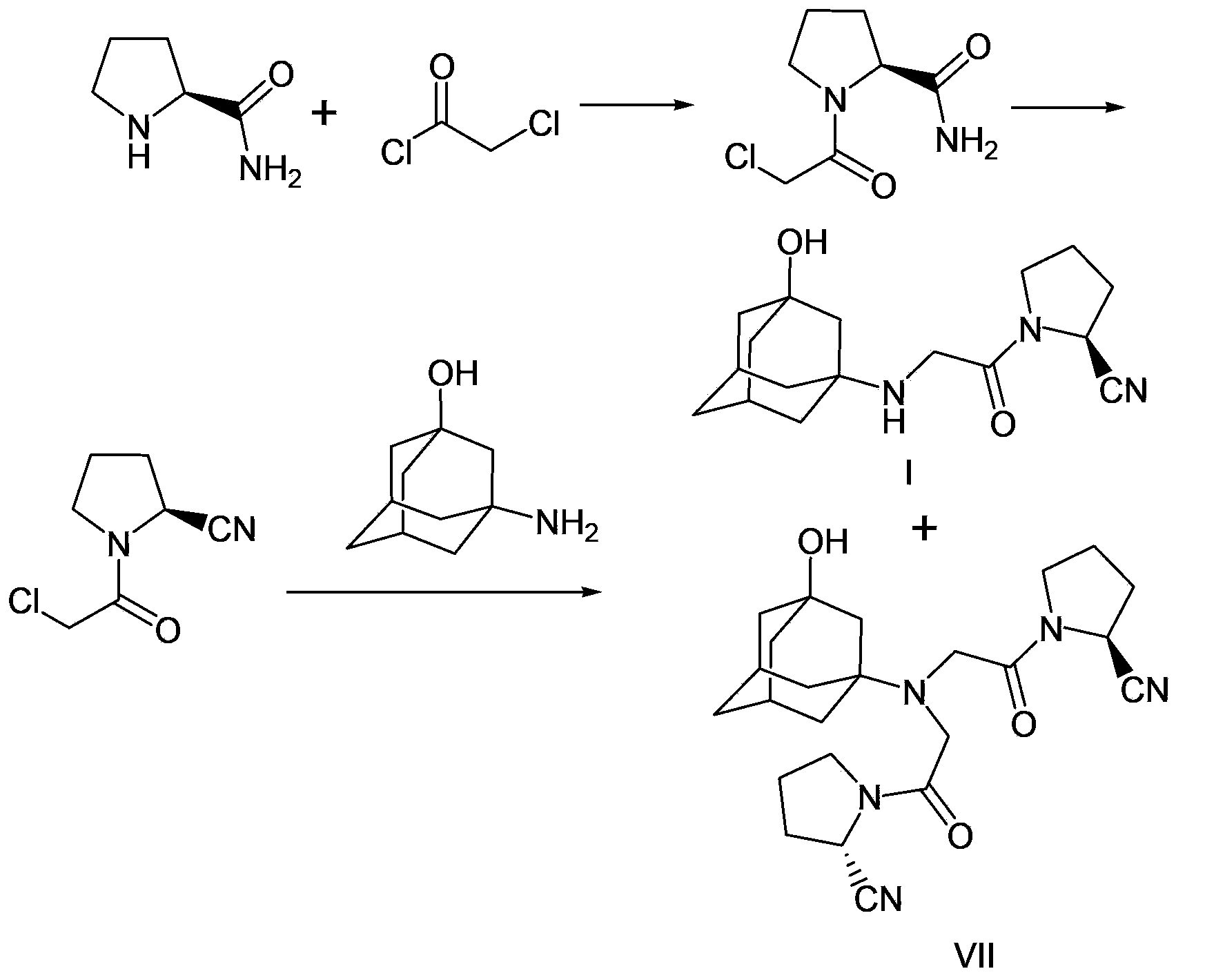
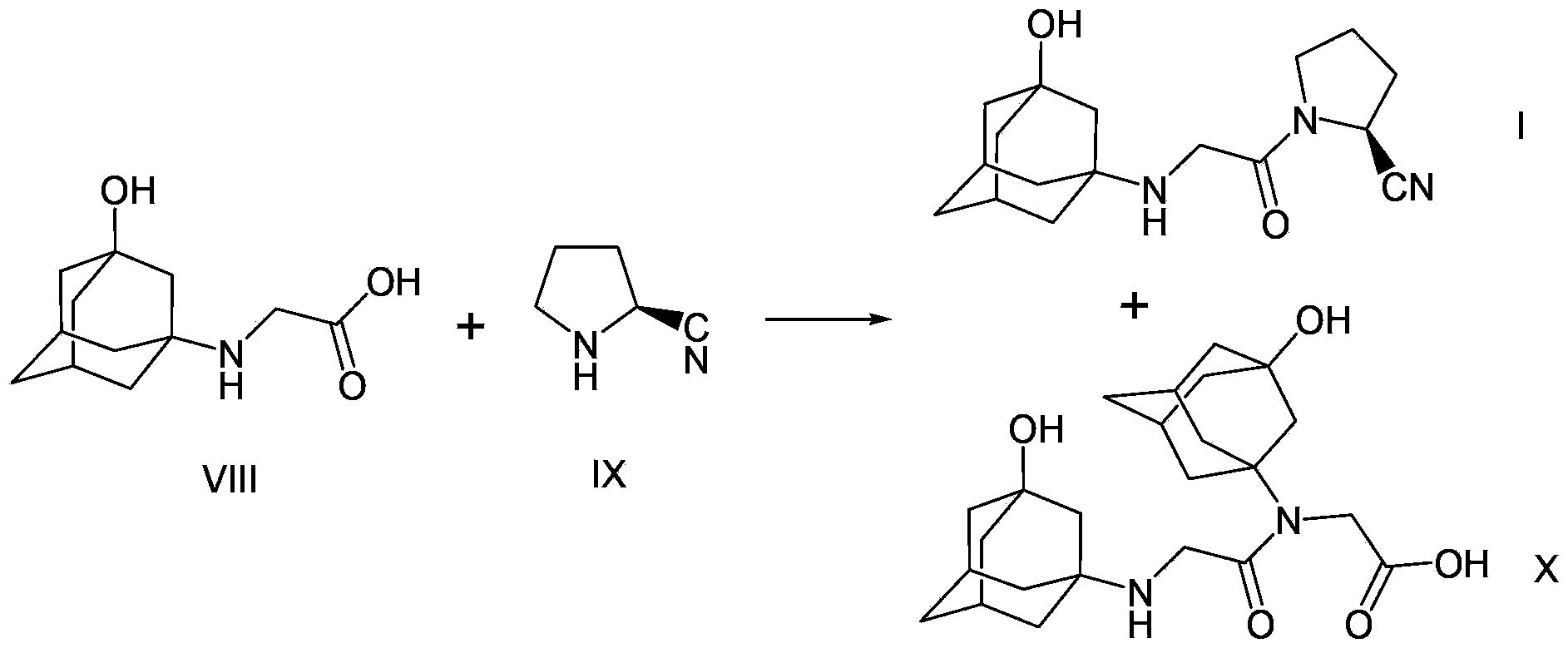
Preparation method of vildagliptin
A technology of compound and solvent, which is applied in the field of preparation of Vildagliptin, can solve the problems such as the decrease of yield, and achieve the effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045]Add 1.68 grams of 3-amino-1-adamantanol, 10 milliliters of anhydrous methanol, and 1.18 grams of benzaldehyde to a 50-milliliter three-necked flask in sequence, heat up to reflux and stir the reaction. TLC (thin-layer chromatography) monitors the reaction until the raw material Transformation is complete, producing intermediates. After cooling down to 0°C, a total of 0.48 g of sodium borohydride was added in batches to the reaction system. After the addition, react at 0°C to 5°C for 1 to 2 hours. After the completion of the reaction was monitored by TLC (thin layer chromatography), the solvent was removed by rotary evaporation. 20 ml of water and 20 ml of ethyl acetate were added to the residue, and the layers were separated to obtain an organic phase. Then use 10 ml of ethyl acetate to extract the aqueous phase three times, combine the organic phases, wash once with 20 ml of saturated brine, dry over anhydrous sodium sulfate, concentrate to remove the solv...
Embodiment 2
[0050]
[0051] 0.26 grams of the product prepared in Example 1 (amino-protected adamantanol), 0.17 grams of (S)-(1)-(2-chloroacetyl)-pyrrolidine-2-carbonitrile, 0.13 gram of diisopropylethylamine, 0.017 gram of potassium iodide, nitrogen replacement several times, 2 milliliters of dichloromethane was added, the temperature was raised to reflux, and the reaction was stirred for 6 hours. The completion of the reaction was monitored by TLC. After cooling to room temperature, it was filtered, and the filtrate was spin-dried, and purified by silica gel column chromatography to obtain the target product with an ESI-MS value of 394.2 (M+1).
Embodiment 3
[0053]
[0054] 0.26 grams of the product prepared in Example 1 (amino-protected adamantanol), 0.17 grams of (S)-(1)-(2-chloroacetyl)-pyrrolidine-2-carbonitrile, 0.11 1 g of sodium carbonate, 0.01 g of sodium bromide, and several times of nitrogen replacement, 2 ml of acetonitrile was added, heated to reflux, and stirred for 6 hours. The completion of the reaction was monitored by TLC. After cooling to room temperature, it was filtered, and the filtrate was spin-dried, and purified by silica gel column chromatography to obtain the target product with an ESI-MS value of 394.2 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com