Novel synthesis method for iopromide
A technology of iopromide and a new method, applied in the field of organic preparation, can solve the problems of unfavorable large-scale industrial production, high production cost, expensive raw materials, etc., and achieve the effects of easy control of product quality, few by-products, and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
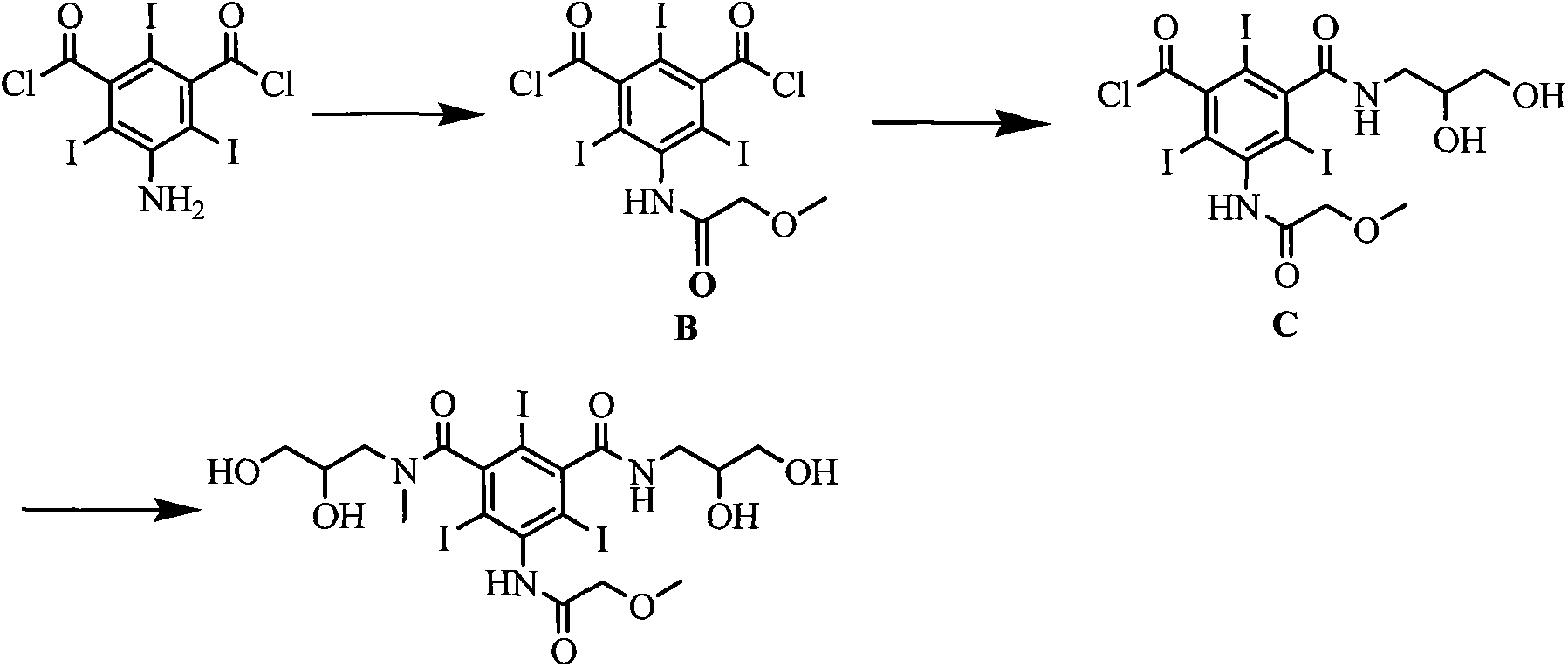
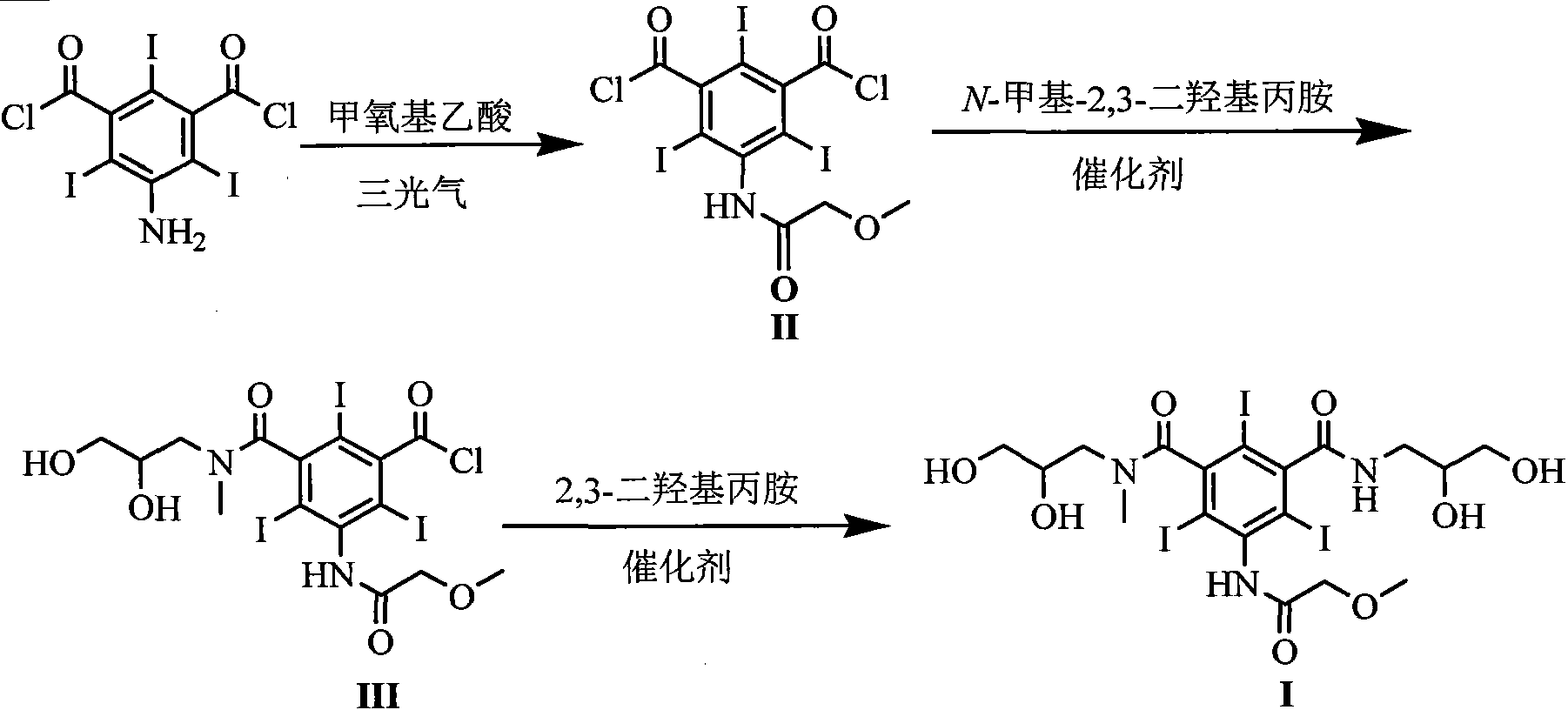
[0025] Embodiment 1 The preparation of formula (II) compound 5-[(2-methoxy)acetamido]-2,4,6-triiodoisophthaloyl chloride
[0026] Add 300mmol of methoxyacetic acid, 110mmol of triphosgene and 300mL of dichloromethane into the reaction flask, stir under reflux for 3h, evaporate the solvent under reduced pressure, cool the reaction flask to 15-20°C and add 200mmol of 5-amino-2,4 , 6-triiodoisophthaloyl chloride and 150mL DMAC were added, and stirring was continued at this temperature for 5h. 700 mL of dichloromethane and 400 mL of water were added and stirred for 0.5 h. Separate the organic layer, wash the organic layer with saturated aqueous sodium bicarbonate solution and distilled water, dry with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and obtain a solid. Yield 90%.
Embodiment 2
[0027] Example 2 Formula (III) compound 5-[(2-methoxy)acetamido]-3-(2,3-dihydroxy-N-methylpropylcarbamoyl)-2,4,6-tri Preparation of iodobenzoyl chloride
[0028] With 100mmol formula (II) compound, 100mmol solid base catalyst ZrO 2 -Cr 2 o 3 and 200mL DMAC were added to the reaction flask, the temperature of the reaction system was lowered to 10-15°C, 90mmol N-methyl-2,3-dihydroxypropylamine was slowly added dropwise, and the reaction was stirred at this temperature for 5h after the drop was completed. The reaction temperature is raised to room temperature, the catalyst is filtered, and the catalyst can be reused after drying. The solvent was evaporated under reduced pressure, 400 mL of dichloromethane was added to the residue, and stirred for 0.5 h. Filter and collect the precipitated solid, add 300mL tetrahydrofuran to the solid, and stir at room temperature for 0.5h. The precipitated solid was filtered and dried to obtain the compound of formula (III) with a yield of 8...
Embodiment 3
[0029] The preparation of embodiment 3 formula (I) compound iopromide
[0030] Add 100mmol of the compound of formula (III), 120mmol of sodium carbonate, 100mmol of 2,3-dihydroxypropylamine and 150mL of DMAC into the reaction flask, stir at room temperature for 2h, evaporate the solvent under reduced pressure, add 400mL of water to the residue, and wash with saturated sodium hydroxide solution After adjusting to weak alkalinity, it was eluted with a cation exchange column and an anion exchange column in sequence. The eluate from the anion exchange column was collected, and the water was distilled off under reduced pressure to obtain a white solid, which was dried under reduced pressure at 70°C to obtain the compound of formula (I) with a yield of 92% and a purity of 99.7% as measured by high pressure liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com