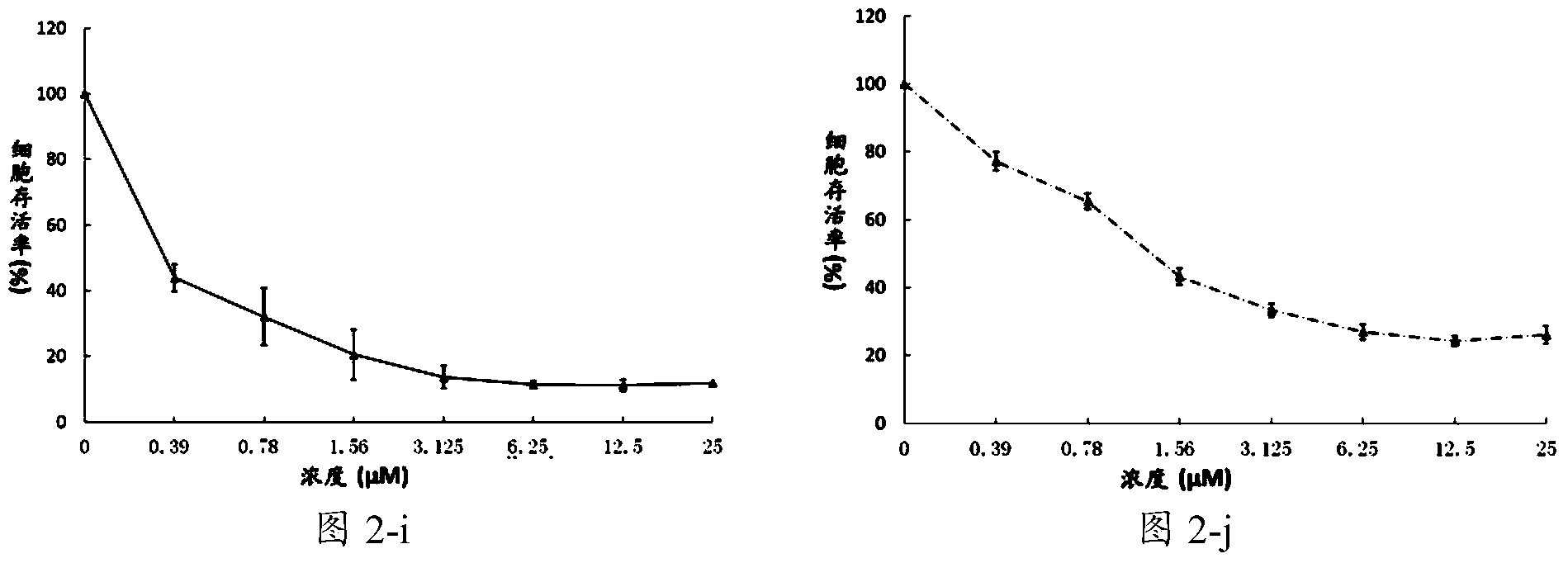Compound and preparation method and application thereof
A compound and drug technology, applied in the field of medicine, can solve problems such as drug resistance and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
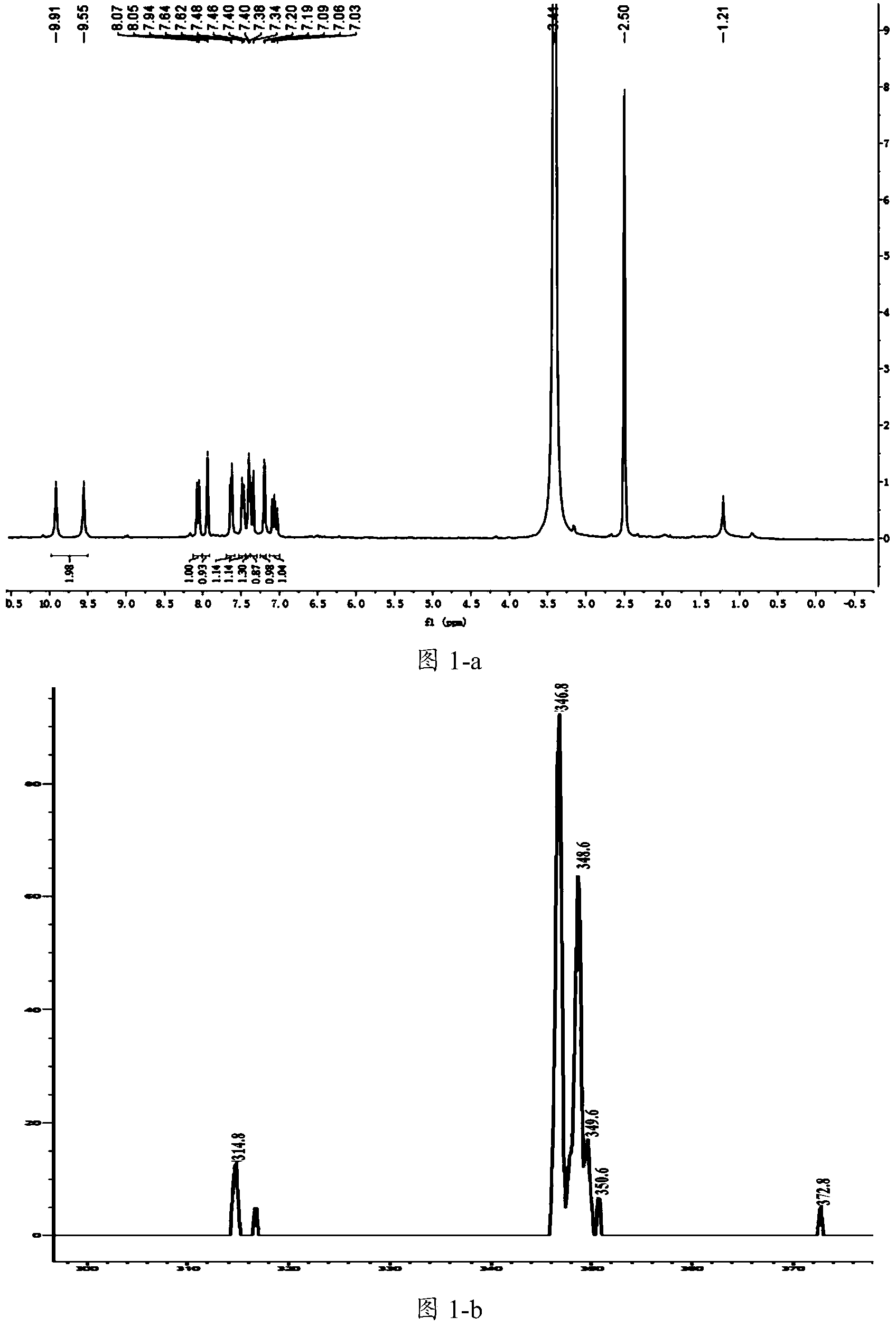
[0125] The preparation of embodiment 1 compound C98
[0126] 1. Dissolve the compound of structure shown in formula VII (2g, 8.3mmol / L, 1.0eq), malonic acid (1.3g, 12.4mmol / L, 1.5eq) in 20ml of anhydrous pyridine, and drop into morpholine under stirring (72.28mg, 0.8mmol / L, 0.1eq), stirred under reflux at 80°C for 24h, after the reaction was completed, 2mol HCl was added to the reaction system, a large amount of solids precipitated, filtered, the filter cake was washed with water, a small amount of EA, and the filter cake was treated with an infrared lamp After drying, the obtained product 2 is a mixture, and the pale yellow solid is 2.4 g of the compound of formula VIII, which can be directly used in subsequent reactions.
[0127] Characterization of the structural compound shown in formula VIII: MS: [M-H]-: Found m / z280.8Calcd m / z281.0
[0128] 2. Suspend the compound of formula VIII (2.4g, 8.48mmol / L, 1.0eq) in the mixed solution of MeOH and DMSO, slowly add SOCl 2 And ad...
Embodiment 2
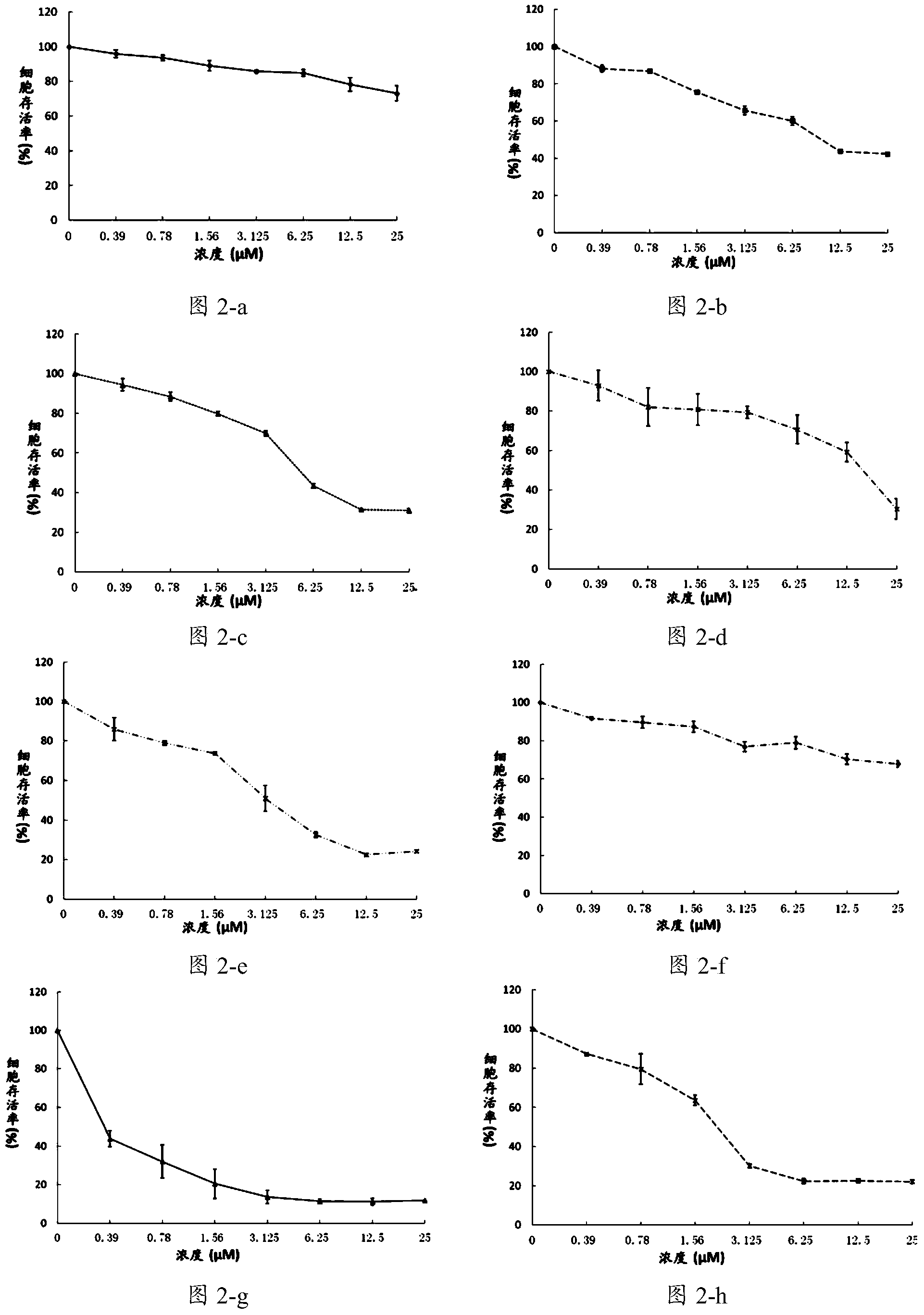
[0139] Example 2 Compound C98 inhibits the growth of multiple myeloma cells and leukemia cells
[0140] Culture a variety of multiple myeloma cells: H929, JJN3, KMS11, KMS18, LP1, MM.1S, OCI-MY5, OPM2, RPMI-8226, U266; leukemia cells: K562, Jurkat, AML-2, NB-4, THP-1.
[0141] Treated with different concentrations of C98, the concentrations of C98 were 0.39μmol / L, 0.78μmol / L, 1.56μmol / L, 3.125μmol / L, 6.25μmol / L, 12.5μmol / L, 25μmol / L. After 72 hours of treatment, cell viability was analyzed by MTS / PMS staining.
[0142] figure 2 The effects of different concentrations of C98 compounds on multiple myeloma cells are shown. The results show that C98 has an inhibitory effect on the growth and proliferation of multiple myeloma in a concentration-dependent manner.
[0143] image 3 The effect of different concentrations of C98 compounds on leukemia cells, the results show that C98 has an inhibitory effect on the proliferation and growth of leukemia cells in a concentration-depen...
Embodiment 3
[0145] Example 3 Compound C98 induces apoptosis of multiple myeloma cells
[0146] Culture LP1, OPM2, OCI-MY5, JJN3 cells.
[0147] Treated with different concentrations of C98, the concentration of C98 was 0 μmol / L, 2.5 μmol / L, 5 μmol / L, and the cells were collected after 24 hours of treatment, and Annexin V-FITC (labeled apoptotic cells) and PI (propidium iodide) were used to treat cells. ) double staining, and Annexin V was detected by flow cytometry + percentage of cells. The result is as Figure 4 and as shown in Table 1:
[0148] Table 1 Effects of different concentrations of C98 compounds on apoptosis of myeloma cells
[0149]
[0150] The results showed that: C98 can effectively induce the apoptosis of multiple myeloma cells, and with the increase of C98 drug concentration, the proportion of cells undergoing apoptosis also gradually increased. It shows that the apoptosis rate is concentration-dependent with C98.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com