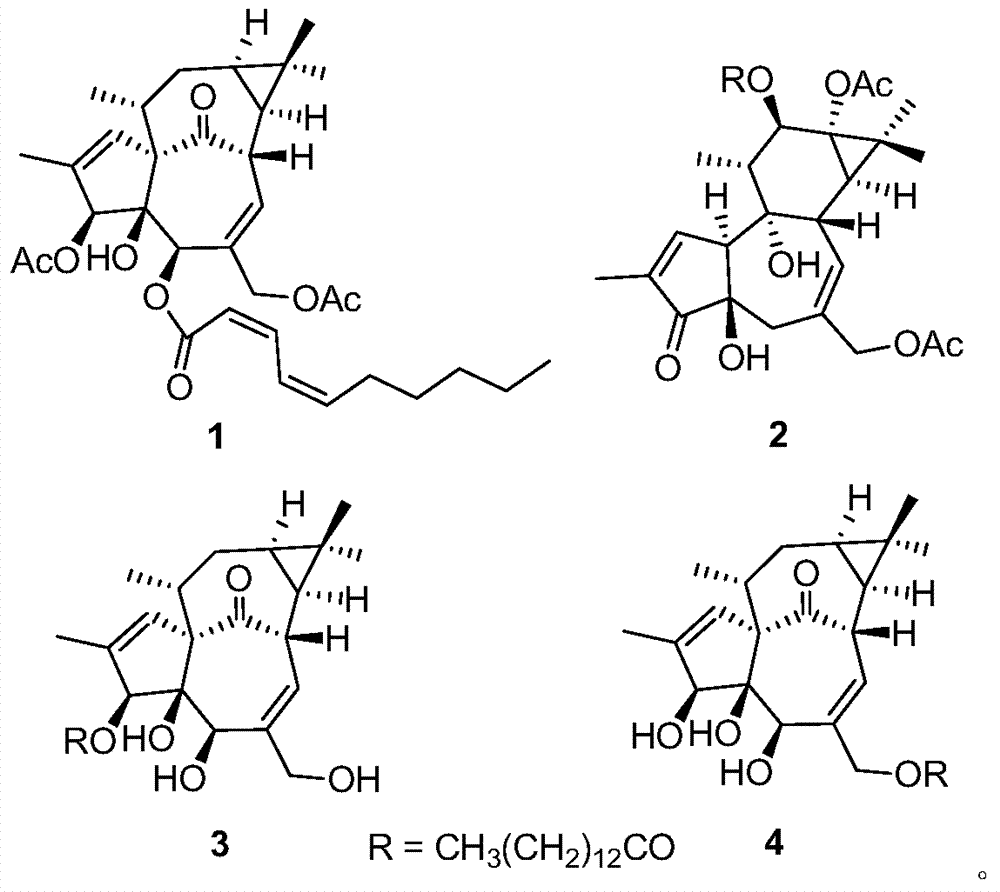
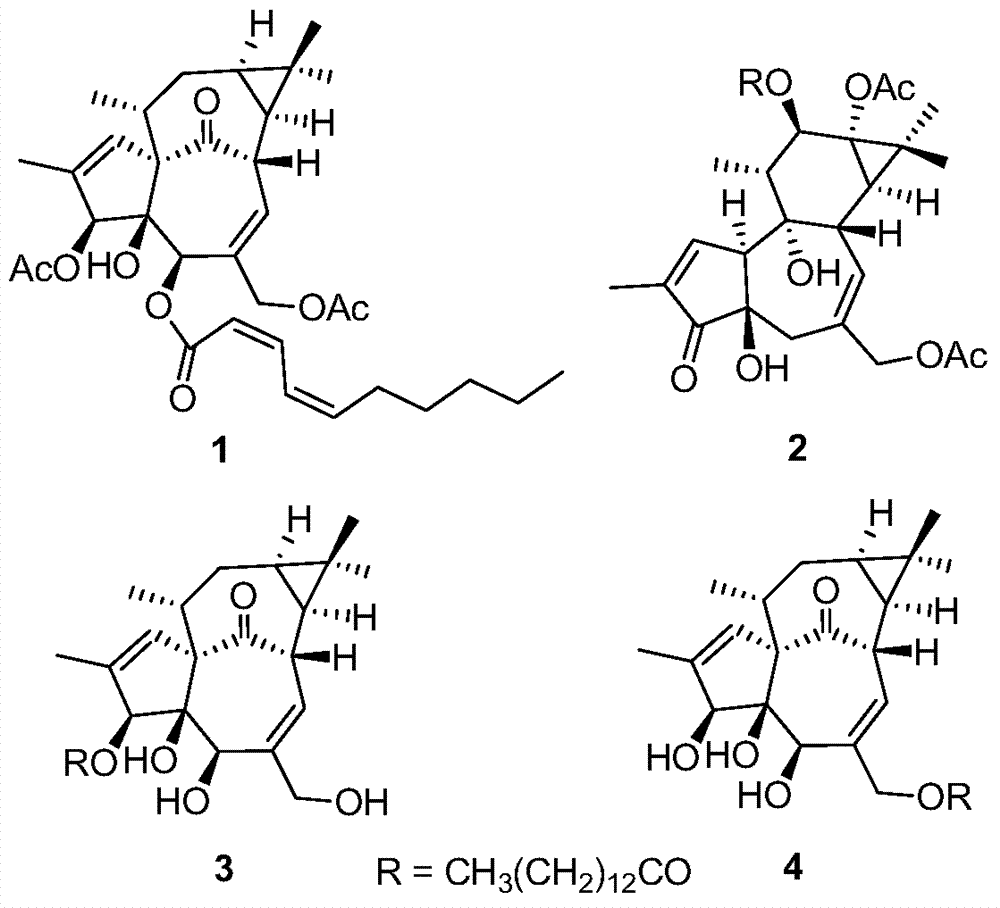
Diterpenoid compounds in euphorbia pekinensis, medicine composition thereof, and application of same in pharmacy
A technology of compounds and diterpenoids, applied in the direction of drug combination, separation/purification of carboxylic acid esters, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of diterpenoids:
[0028] The dried whole plant of Euphorbia euphorbia (1.5kg) was pulverized, cold-soaked in 90% ethanol (4×6L) at room temperature for 24 hours, filtered, the extracts were combined, and the ethanol was recovered under reduced pressure to obtain a crude extract. Ester was extracted 3 times to obtain 103 g of ethyl acetate extract, which was eluted with polyamide and MCI series methanol-water (20:80 to 100:0) gradient, and the same fractions were combined to obtain 5 fractions (F1-F5) . Take F4 and get 4 small fractions (F4a-F4d) by gradient elution of silica gel column chloroform-ethyl acetate (10:1 to 3:2), and take F4c and go through Sephadex LH-20 column chromatography (chloroform:methanol=1:1) After purification, compound 1 (1.3 mg) was semi-prepared by HPLC (methanol:water=82:18). Take F5 through silica gel column petroleum ether-ethyl acetate (10:1 to 2:3) gradient elution to obtain 3 small fractions (F5a-F5c), take F5a and semi-pre...
Embodiment 2
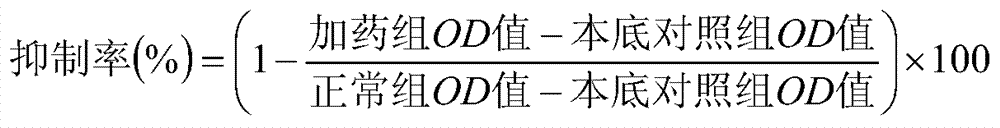
[0038] The pharmacological test of diterpenoid compound (1-4) of the present invention:
[0039] Experimental materials: DMEM medium, newborn calf serum, trypsin, MTT, DMSO, human lung adenocarcinoma cell line A549, liver cancer cell line Hep G2, human cervical cancer cell line Hela, CO 2 Incubator, ultra-clean bench, microplate reader, inverted microscope, cell culture flask, 96-well cell culture plate.
[0040] experimental method:
[0041] 1. Medium preparation: GIBCO DMEM liquid medium, refrigerated at 4°C for later use.
[0042] 2. Inactivation of calf serum: 200mL super newborn calf serum, take it out of the -40℃ refrigerator and thaw at room temperature, and bathe in 56℃ water for 30min. After cooling, aliquot them into 15mL centrifuge tubes and freeze them in a -40°C refrigerator for later use.
[0043] 3. Complete culture medium: 95 mL of DMEM medium, add 5 mL of inactivated newborn calf serum, and mix well.
[0044] 4. Sample stock solution: Accurately measure 10.0...
Embodiment 3
[0063] Preparation of Injections
[0064] Prepare diterpene compound earlier by the method for embodiment 1, mix it with 20% polyoxyethylene ether castor oil, dissolve into Suspension injection made in water.
[0065] It is also possible to dissolve the diterpene compounds prepared by the method of Example 1 with a small amount of DMSO, add water for injection as usual, perform fine filtration, potting, and sterilize to make an injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com