Application of curcumin analog S5 containing thiapyrone structure in preparation of anti-inflammation drugs
A technology of curcumin analogues and thiopyrones, which can be used in medical preparations containing active ingredients, anti-inflammatory agents, drug combinations and other directions, and can solve the problems of weak stability of curcumin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of Compound S5
[0023] At room temperature, 10 mmol of vanillin was dissolved in 12 mL of ethanol, and then 5 mmol of thiopyrone was added thereto. After reacting at room temperature for half an hour, HCl gas was passed into it until TLC detected that the starting point disappeared. Then the reaction mixture was poured into 25 mL of ice water, and a yellow precipitate was produced. After filtration, the residual solid was washed with water and directly carried out to the next reaction without further purification. Dissolve the residual solid in 10 mL of tetrahydrofuran, add 3 mL of triethylamine, stir for half an hour, then add corresponding 10 mmol of isobutyryl chloride to it, after the reaction is complete, concentrate, and pass through a silica gel column to obtain compound S5 with a purity greater than 98%. target compound.
[0024] [(1Z,1'Z)-(4-Oxo-2H-thiopyran-3,5(4H,6H)-diylidene)bis(methylidene)]bis(2-methoxy-4,1-phenylene)bis(2- methylpropanoate)...
Embodiment 2
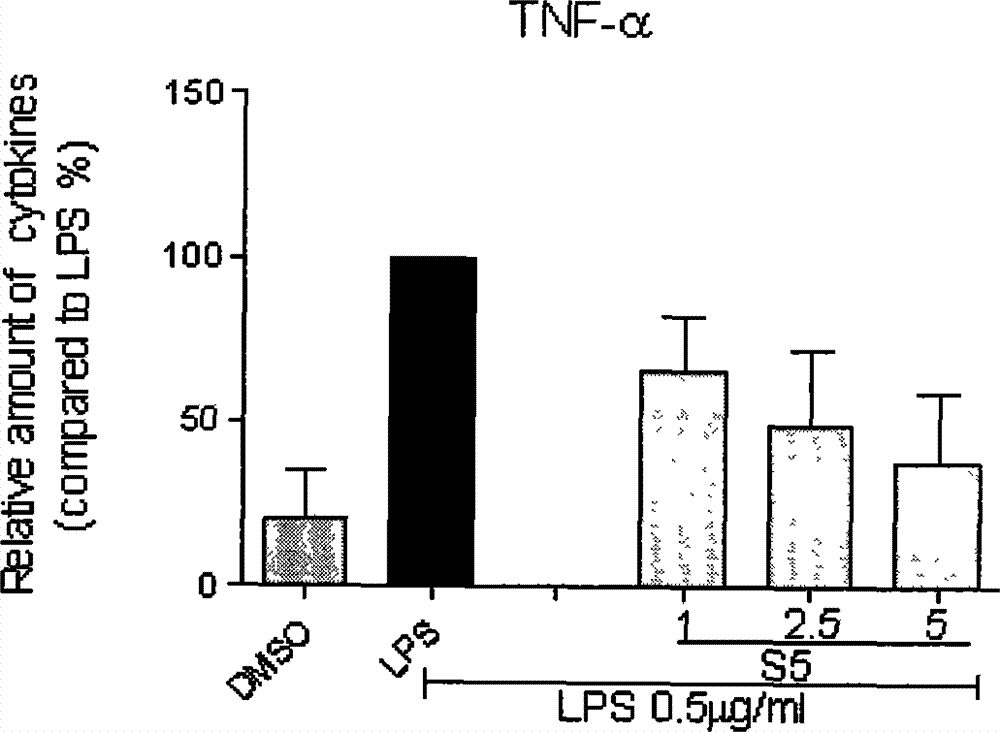
[0026] Compound S5 inhibits the release of inflammatory factors (TNF-α and IL-6) from macrophages stimulated by LPS
[0027] The method of inhibiting the release of inflammatory factors by LPS-stimulated macrophages using compounds, the detailed steps are as follows: 1.2×10 6 RAW264.7 macrophages were cultured with DMEM medium at 37°C. After 24 hours, the medium was renewed, and the compound to be tested (the final concentration was 10 μM) was added for pretreatment for 2 hours, and then continued to be treated with 0.5 μg / mL LPS. After 22 hours, the culture fluid was collected, and the contents of TNF-α and IL-6 were detected by ELISA; the cells were collected to detect the total protein concentration. The -6 content was calibrated to 100; each compound was tested three times, and the average value and error value were calculated. During the test, the positive drug curcumin (cur) was used as a control. The inhibitory activities of compound S5 on the release of IL-6 and TNF-...
Embodiment 3
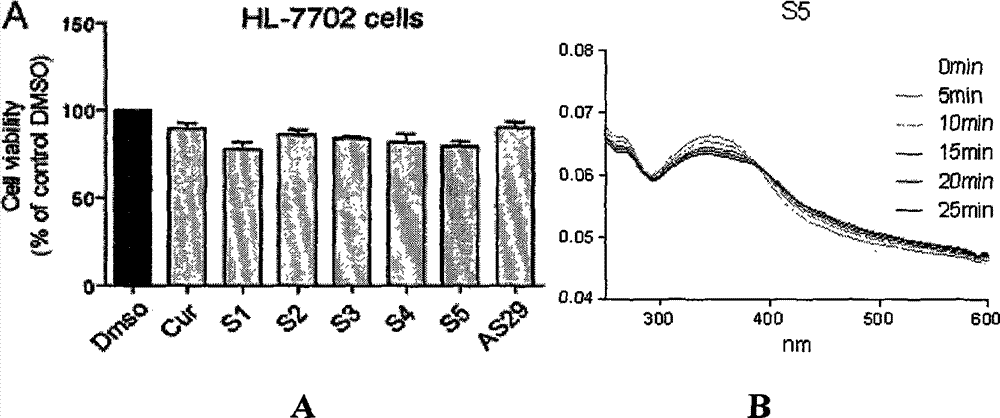
[0030] Toxicity of compound S5 to normal human liver cells HL-7702 and the stability test of its compound
[0031] After being treated with 10 μM compound S5 for 24 hours, the cell survival rate of human normal hepatocyte cells HL-7702 was detected by MTT (thiazolium blue), which was used to characterize the activity of the compound on hepatocyte HL-7702 ( image 3 A); Compound S5 was dissolved in DMSO to prepare a 10 μM solution. The absorbance of the compound at 0, 5, 10, 15, 20, and 25 min was detected in the wavelength range of 250-600 nm. Because the intensity of absorbance is directly proportional to the content or concentration of the compound in the solution, this parameter is used to characterize the stability of the compound ( image 3 B).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com