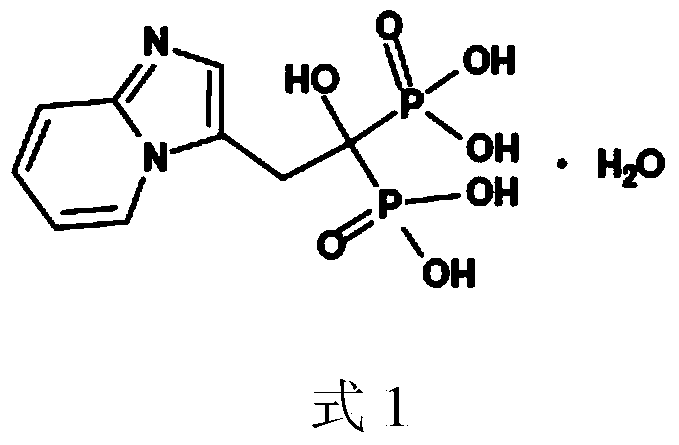
Minodronic acid medicinal composition and preparation method thereof
A minodronic acid and composition technology, applied in the field of minodronic acid pharmaceutical composition and its preparation, can solve the problems of difficult uniform mixing, poor patient compliance, easy aggregation, etc., and achieve improved content uniformity and quality control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Investigation on the appropriate ratio and dosage of lactose and starch in the filler
[0036] Table 1 Prescription Screening-Investigation of Filler Ratio Screening Process
[0037]
[0038] Note: the starch in the binder starch slurry accounts for 15% by mass of the total starch in the minodronic acid pharmaceutical composition, and the starch in the binder starch slurry accounts for 8% by mass of water.
[0039] As shown in Table 1, four groups of minodronic acid pharmaceutical compositions were prepared according to prescriptions 1, 2, 3 and 4, and their quality was inspected, and the results are shown in Table 2.
[0040] Table 2 Prescription Screening - Results of Investigation of Filler Ratio
[0041]
[0042] Result analysis: the main difference between prescription 1, prescription 2, prescription 3 and prescription 4 is the proportion of lactose and starch used in fillers. The proportions of lactose used in prescriptions 1, 2, 3 and 4 are 90%,...
Embodiment 2
[0043] Embodiment 2: the investigation of adhesive
[0044] On the basis of prescription 2, combined with the common use of excipients, the analysis of the original research product, the excipients that may be used as binders in the prescription are hydroxypropyl cellulose and starch, and different varieties and concentrations of hydroxypropyl cellulose and starch are investigated. The effect of starch slurry as a binder on product quality. Select different concentrations of starch slurry and hydroxypropyl cellulose as the binder, and the starch or hydroxypropyl cellulose in the binder starch slurry accounts for 15% by mass of the total starch in the minodronic acid pharmaceutical composition %,as shown in Table 3.
[0045] Table 3 Prescription Screening - Investigation of Adhesive Use Single Factor
[0046]
[0047]
[0048] The inspection results are shown in Table 4.
[0049] Table 4 Prescription Screening - Results of Adhesive Use Single Factor Investigation
[0...
Embodiment 3
[0053] Embodiment 3: the dosage investigation of lubricant magnesium stearate
[0054] On the basis of prescription 8, investigate the usage dosage of lubricant magnesium stearate, investigate according to the commonly used amount of magnesium stearate in preparation as lubricant 1%, 3%. The specific formula is shown in Table 5.
[0055] Table 5 Prescription Screening-Lubricant Use Single Factor Investigation
[0056]
[0057] Table 6 Prescription Screening - Results of Lubricant Use Single Factor Investigation
[0058]
[0059] Result analysis: Prescription 10 adopts the wet tableting process, 8% starch slurry is used as a binder, and the amount of lubricant used is 3%. The disintegration time limit is relatively small, and prescription 8 is still selected to continue the research.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com